An inactive pool of GSK-3 at the leading edge of growth cones is implicated in Semaphorin 3A signaling
- PMID: 11956225
- PMCID: PMC2199247
- DOI: 10.1083/jcb.200201098
An inactive pool of GSK-3 at the leading edge of growth cones is implicated in Semaphorin 3A signaling
Abstract
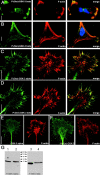
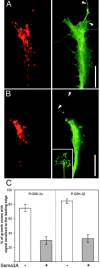
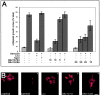
Glycogen synthase kinase (GSK)-3 is a serine/threonine kinase that has been implicated in several aspects in embryonic development and several growth factor signaling cascades. We now report that an inactive phosphorylated pool of the enzyme colocalizes with F-actin in both neuronal and nonneuronal cells. Semaphorin 3A (Sema 3A), a molecule that inhibits axonal growth, activates GSK-3 at the leading edge of neuronal growth cones and in Sema 3A-responsive human breast cancer cells, suggesting that GSK-3 activity might play a role in coupling Sema 3A signaling to changes in cell motility. We show that three different GSK-3 antagonists (LiCl, SB-216763, and SB-415286) can inhibit the growth cone collapse response induced by Sema 3A. These studies reveal a novel compartmentalization of inactive GSK-3 in cells and demonstrate for the first time a requirement for GSK-3 activity in the Sema 3A signal transduction pathway.
Figures


References
-
- Aizawa, H., S. Wakatsuki, A. Ishii, K. Moriyama, Y. Sasaki, K. Ohashi, Y. Sekine-Aizawa, A. Sehara-Fujisawa, K. Mizuno, Y. Goshima, et al. 2001. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat. Neurosci. 4:367–373. - PubMed
-
- Bagnard, D., C. Vaillant, S.T. Khuth, N. Dufay, M. Lohrum, A.W. Puschel, M.F. Belin, J. Bolz, and N. Thomasset. 2001. Semaphorin 3A-vascular endothelial growth factor-165 balance mediates migration and apoptosis of neural progenitor cells by the recruitment of shared receptor. J. Neurosci. 21:3332–3341. - PMC - PubMed
-
- Baum, L., R. Seger, J.R. Woodgett, S. Kawabata, K. Maruyama, M. Koyama, J. Silver, and T. Saitoh. 1995. Overexpressed tau protein in cultured cells is phosphorylated without formation of PHF: implication of phosphoprotein phosphatase involvement. Brain Res. Mol. Brain Res. 34:1–17. - PubMed
-
- Coghlan, M.P., A.A. Culbert, D.A. Cross, S.L. Corcoran, J.W. Yates, N.J. Pearce, O.L. Rausch, G.J. Murphy, P.S. Carter, L. Roxbee Cox, et al. 2000. Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem. Biol. 7:793–803. - PubMed

