The plasma kallikrein-kinin system counterbalances the renin-angiotensin system
- PMID: 11956236
- PMCID: PMC150954
- DOI: 10.1172/JCI15490
The plasma kallikrein-kinin system counterbalances the renin-angiotensin system
Figures

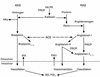
Comment on
-
Increased vascular permeability in C1 inhibitor-deficient mice mediated by the bradykinin type 2 receptor.J Clin Invest. 2002 Apr;109(8):1057-63. doi: 10.1172/JCI14211. J Clin Invest. 2002. PMID: 11956243 Free PMC article.
References
-
- Shariat-Madar Z, Mahdi F, Schmaier AH. Mapping the cell binding site on cytokeratin 1. J Biol Chem. 1999;274:7137–7145. - PubMed
-
- Herwald H, Dedio J, Kellner R, Loos M, Muller-Esterl W. Isolation and characterization of the kininogen-binding protein p33 from endothelial cells. J Biol Chem. 1996;271:13040–13047. - PubMed
-
- Joseph K, Ghebrehiwet B, Peerschke EIB, Reid KBM, Kaplan AP. Identification of the zinc-dependent endothelial cell binding protein for high molecular weight kininogen and factor XII: identity with the receptor that binds to the globular “heads” of C1q (gC1q-R) Proc Natl Acad Sci USA. 1996;93:8552–8557. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

