Increased vascular permeability in C1 inhibitor-deficient mice mediated by the bradykinin type 2 receptor
- PMID: 11956243
- PMCID: PMC150945
- DOI: 10.1172/JCI14211
Increased vascular permeability in C1 inhibitor-deficient mice mediated by the bradykinin type 2 receptor
Abstract
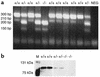
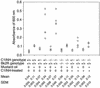
Heterozygosity for C1 inhibitor (C1INH) deficiency results in hereditary angioedema. Disruption of the C1INH gene by gene trapping enabled the generation of homozygous- and heterozygous-deficient mice. Mating of heterozygous-deficient mice resulted in the expected 1:2:1 ratio of wild-type, heterozygous, and homozygous-deficient offspring. C1INH-deficient mice showed no obvious phenotypic abnormality. However, following injection with Evans blue dye, both homozygous and heterozygous C1INH-deficient mice revealed increased vascular permeability in comparison with wild-type littermates. This increased vascular permeability was reversed by treatment with intravenous human C1INH, with a Kunitz domain plasma kallikrein inhibitor (DX88), and with a bradykinin type 2 receptor (Bk2R) antagonist (Hoe140). In addition, treatment of the C1INH-deficient mice with an angiotensin-converting enzyme inhibitor (captopril) increased the vascular permeability. Mice with deficiency of both C1INH and Bk2R demonstrated diminished vascular permeability in comparison with C1INH-deficient, Bk2R-sufficient mice. These data support the hypothesis that angioedema is mediated by bradykinin via Bk2R.
Figures





Comment in
-
The plasma kallikrein-kinin system counterbalances the renin-angiotensin system.J Clin Invest. 2002 Apr;109(8):1007-9. doi: 10.1172/JCI15490. J Clin Invest. 2002. PMID: 11956236 Free PMC article. Review. No abstract available.
References
-
- Sim RB, Reboul A, Arlaud GJ, Villiers CL, Colomb MG. Interaction of 125I-labeled complement components C1r and C1s with protease inhibitors in plasma. FEBS Lett. 1979;97:111–115. - PubMed
-
- Forbes C, Pensky J, Ratnoff O. Inhibition of activated Hageman factor and activated plasma thromboplastin antecedent by purified C1 inactivator. J Lab Clin Med. 1970;76:809–815. - PubMed
-
- Gigli I, Mason JW, Colman RW, Austen KF. Interaction of plasma kallikrein with the C1 inhibitor. J Immunol. 1970;104:574–581. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

