NO donors potentiate the beta-adrenergic stimulation of I(Ca,L) and the muscarinic activation of I(K,ACh) in rat cardiac myocytes
- PMID: 11956332
- PMCID: PMC2290242
- DOI: 10.1113/jphysiol.2001.012929
NO donors potentiate the beta-adrenergic stimulation of I(Ca,L) and the muscarinic activation of I(K,ACh) in rat cardiac myocytes
Abstract
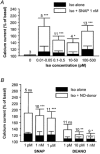
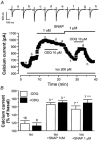
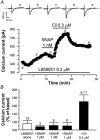
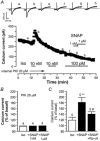
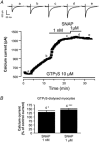
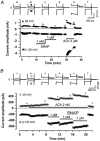
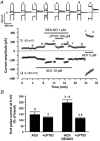
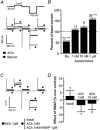
The effects of nitric oxide (NO) donors on the L-type Ca(2+) current (I(Ca,L)) and the muscarinic activated K(+) current (I(K,ACh)) were studied in isolated rat cardiac myocytes. The nitrosothiol S-nitroso-N-acetyl-D,L-penicillamine (SNAP, 1 pM-1 microM) strongly potentiated the stimulation of the I(Ca,L) elicited by subthreshold concentrations of isoprenaline (Iso, 0.1-0.5 nM) in ventricular myocytes. The effect of SNAP was mimicked by 2-(N,N-diethylamino)-diazenolate-2-oxide (DEANO, 1 pM-1 nM), a NONOate that spontaneously releases NO in a pH-controlled manner, and was blunted by 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (100 microM), a NO trap. 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxaline-1-one (10 microM), a guanylyl cyclase inhibitor, did not alter the effect of SNAP. SNAP (1 pM-1 microM) did not modify the effect of L858051 (0.1-0.3 microM), a forskolin analogue that activates adenylyl cyclase, on I(Ca,L) and did not enhance the basal I(Ca,L) in the presence of rolipram (1 microM), a phosphodiesterase type 4 inhibitor. Superfusion with Rp-CPT-cAMPS (500 microM), or internal dialysis with cAMP-dependent protein kinase (cA-PK) inhibitory peptide (PKI; 20 microM), inhibitors of the cA-PK, blunted the effect of SNAP (1 nM and 1 microM) on the Iso-stimulated (1-100 pM) I(Ca,L). SNAP (1 nM and 1 microM) potentiated the threshold stimulation of I(Ca,L) elicited by internal GTP-gammaS (10 microM), a non-hydrolysable analogue of GTP. SNAP (1 pM-1 microM) and DEANO (1 microM) potentiated the stimulation of I(K,ACh) elicited by low concentrations of ACh (1-2 nM) in rat atrial myocytes. The threshold stimulation of I(K,ACh) elicited by internal 5'-guanylylimidodiphosphate (10 microM) was also potentiated by NO donors. SNAP (1 microM) did not modify I(K,ACh) reconstituted in human embryonic kidney 293 cells, in the absence or in the presence of ACh (1 or 10 nM). Taken together, these data suggest that NO is a cGMP-independent modulator of G-protein-coupled muscarinic and beta-adrenergic receptor actions on cardiac ion channels. Although this action of NO seemed to occur at the level of G proteins, it appeared to require a component distinct from receptors, G proteins or their effectors.
Figures



References
-
- Abi-Gerges N, Eschenhagen T, Hove-Madsen L, Fischmeister R, Méry PF. Methylene blue is a muscarinic antagonist in cardiac myocytes. Molecular Pharmacology. 1997;52:482–490. - PubMed
-
- Abi-Gerges N, Fischmeister R, Méry PF. Regulation of acetylcholine-activated potassium current by nitric oxide in isolated rat atrial myocytes. Biophysical Journal. 2001b;80:628a–629a.
-
- Adam L, Bouvier M, Jones TLZ. Nitric oxide modulates β2-adrenergic receptor palmitoylation and signaling. Journal of Biological Chemistry. 1999;274:26337–26343. - PubMed
-
- Akaike T, Yoshida M, Miyamoto Y, Sato K, Kohno M, Sasamoto K, Miyazaki K, Ueda S, Maeda H. Antagonistic action of imidazolineoxyl N-oxides against endothelium-derived relaxing factor/. NO through a radical reaction. Biochemistry. 1993;32:827–832. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

