Compatible osmolytes modulate the response of porcine endothelial cells to hypertonicity and protect them from apoptosis
- PMID: 11956339
- PMCID: PMC2290260
- DOI: 10.1113/jphysiol.2001.013395
Compatible osmolytes modulate the response of porcine endothelial cells to hypertonicity and protect them from apoptosis
Abstract
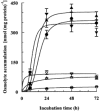
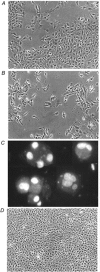
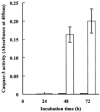
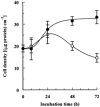
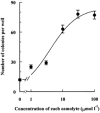
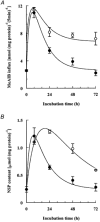
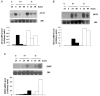
Porcine pulmonary arterial endothelial cells accumulated myo-inositol and taurine, as well as betaine, during adaptation to hypertonic stress. The cells grew and maintained their normal morphology during culture in hypertonic (0.5 osmol (kg H(2)O)(-1)) medium that contained osmolytes such as betaine, myo-inositol or taurine at concentrations close to reported physiological values. The cells did not grow well in hypertonic medium depleted of potential compatible osmolytes. After a few days, cell density decreased by about 50 % and many cells rounded up and detached from the plates, their nuclei showing clear apoptotic morphology. The caspase-3 activity of the cells also increased dramatically under these conditions, but remained negligibly low when betaine and myo-inositol were added to the medium. Addition of betaine and myo-inositol to hypertonic medium depleted of compatible osmolytes increased the number of colonies remaining after 12 days of culture; with each solute at 30-100 micromol l(-1) the number increased about sixfold. In the absence of compatible osmolytes, increased mRNA levels and corresponding activities of betaine/gamma-aminobutyric acid transporter (BGT1) and sodium/myo-inositol transporter (SMIT) induced by hypertonicity remained high after 72 h incubation, whereas they were down regulated in the presence of betaine and myo-inositol. Similarly, the down regulation of the amino acid System A transporter (ATA2) was markedly slowed in the absence of compatible osmolytes. We conclude that these compatible osmolytes at concentrations close to physiological values enable the endothelial cells to adapt to hypertonic stress, protecting them from apoptosis, and also modulate the adaptation process.
Figures

References
-
- Alfieri RR, Petronini PG, Bonelli MA, Caccamo AE, Cavazzoni A, Borghetti AF, Wheeler KP. Osmotic regulation of ATA2 mRNA expression and amino acid transport System A activity. Biochemical and Biophysical Research Communications. 2001;283:174–178. - PubMed
-
- Allen RH, Stabler SP, Lindenbaum J. Serum betaine, N,N-dimethylglycine and N-methylglycine levels in patients with cobalamin and folate deficiency and related inborn errors of metabolism. Metabolism. 1993;42:1448–1460. - PubMed
-
- Bortner CD, Cidlowski JA. Absence of volume regulatory mechanisms contributes to the rapid activation of apoptosis in thymocytes. American Journal of Physiology. 1996;271C:950–961. - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. - PubMed
-
- Burg MB. Molecular basis of osmotic regulation. American Journal of Physiology. 1995;268F:983–996. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

