Intrapulmonary pharmacokinetics of linezolid
- PMID: 11959585
- PMCID: PMC127139
- DOI: 10.1128/AAC.46.5.1475-1480.2002
Intrapulmonary pharmacokinetics of linezolid
Abstract
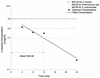
In this study, our objective was to determine the steady-state intrapulmonary concentrations and pharmacokinetic parameters of orally administered linezolid in healthy volunteers. Linezolid (600 mg every 12 h for a total of five doses) was administered orally to 25 healthy adult male subjects. Each subgroup contained five subjects, who underwent bronchoscopy and bronchoalveolar lavage (BAL) 4, 8, 12, 24, or 48 h after administration of the last dose. Blood was obtained for drug assay prior to administration of the first dose and fifth dose and at the completion of bronchoscopy and BAL. Standardized bronchoscopy was performed without systemic sedation. The volume of epithelial lining fluid (ELF) recovered was calculated by the urea dilution method, and the total number of alveolar cells (AC) was counted in a hemocytometer after cytocentrifugation. Linezolid was measured in plasma by a high-pressure liquid chromatography (HPLC) technique and in BAL specimens and AC by a combined HPLC-mass spectrometry technique. Areas under the concentration-time curves (AUCs) for linezolid in plasma, ELF, and AC were derived by noncompartmental analysis. Half-lives for linezolid in plasma, ELF, and AC were calculated from the elimination rate constants derived from a monoexponential fit of the means of the observed concentrations at each time point. Concentrations (means +/- standard deviations) in plasma, ELF, and AC, respectively, were 7.3 +/- 4.9, 64.3 +/- 33.1, and 2.2 +/- 0.6 microg/ml at the 4-h BAL time point and 7.6 +/- 1.7, 24.3 +/- 13.3, and 1.4 +/- 1.3 microg/ml at the 12-h BAL time point. Linezolid concentrations in plasma, ELF, and AC declined monoexponentially, with half-lives of 6.9, 7.0, and 5.7 h, respectively. For a MIC of 4, the 12-h plasma AUC/MIC and maximum concentration/MIC ratios were 34.6 and 3.9, respectively, and the percentage of time the drug remained above the MIC for the 12-h dosing interval was 100%; the corresponding ratios in ELF were 120 and 16.1, respectively, and the percentage of time the drug remained above the MIC was 100%. The long plasma and intrapulmonary linezolid half-lives and the percentage of time spent above the MIC of 100% of the dosing interval provide a pharmacokinetic rationale for drug administration every 12 h and indicate that linezolid is likely to be an effective agent for the treatment of pulmonary infections.
Figures


References
-
- Baldwin, D. R., S. R. Maxwell, D. Honeybourne, J. M. Andrews, J. P. Ashby, and R. Wise. 1991. The penetration of cefpirome into the potential sites of pulmonary infection. J. Antimicrob. Chemother. 28:79-86. - PubMed
-
- Baldwin, D. R., R. Wise, J. M. Andrews, J. P. Ashby, and D. Honeybourne. 1990. Azithromycin concentrations at the sites of pulmonary infection. Eur. Respir. J. 3: 886-890. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

