Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of Raldh2 null mutant mice
- PMID: 11959834
- PMCID: PMC2833017
- DOI: 10.1242/dev.129.9.2271
Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of Raldh2 null mutant mice
Abstract
Retinoid control of vertebrate development depends upon tissue-specific metabolism of retinol to retinoic acid (RA). The RA biosynthetic enzyme RALDH2 catalyzes much, but not all, RA production in mouse embryos, as revealed here with Raldh2 null mutants carrying an RA-responsive transgene. Targeted disruption of Raldh2 arrests development at midgestation and eliminates all RA synthesis except that associated with Raldh3 expression in the surface ectoderm of the eye field. Conditional rescue of Raldh2(-/-) embryos by limited maternal RA administration allows development to proceed and results in the establishment of additional sites of RA synthesis linked to Raldh1 expression in the dorsal retina and to Raldh3 expression in the ventral retina, olfactory pit and urinary tract. Unexpectedly, conditionally rescued Raldh2(-/-) embryos also possess novel sites of RA synthesis in the neural tube and heart that do not correspond to expression of Raldh1-3. RA synthesis in the mutant neural tube was localized in the spinal cord, posterior hindbrain and portions of the midbrain and forebrain, whereas activity in the mutant heart was localized in the conotruncus and sinus venosa. In the posterior hindbrain, this novel RA-generating activity was expressed during establishment of rhombomeric boundaries. In the spinal cord, the novel activity was localized in the floorplate plus in the intermediate region where retinoid-dependent interneurons develop. These novel RA-generating activities in the neural tube and heart fill gaps in our knowledge of how RA is generated spatiotemporally and may, along with Raldh1 and Raldh3, contribute to rescue of Raldh2(-/-) embryos by producing RA locally.
Figures



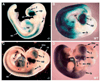



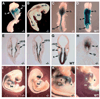
References
-
- Ang HL, Deltour L, Hayamizu TF, Zgombic-Knight M, Duester G. Retinoic acid synthesis in mouse embryos during gastrulation and craniofacial development linked to class IV alcohol dehydrogenase gene expression. J. Biol. Chem. 1996;271:9526–9534. - PubMed
-
- Armstrong RB, Ashenfelter KO, Eckhoff C, Levin AA, Shapiro SS. General and reproductive toxicology of retinoids. In: Sporn MB, Roberts AB, Goodman DS, editors. The Retinoids: Biology, Chemistry and Medicine. 2nd edition. New York: Raven Press, Ltd; 1994. pp. 545–572.
-
- Batourina E, Gim S, Bello N, Shy M, Clagett-Dame M, Srinivas S, Costantini F, Mendelsohn C. Vitamin A controls epithelial/mesenchymal interactions through Ret expression. Nature Genet. 2001;27:74–78. - PubMed
-
- Chapman DL, Garvey N, Hancock S, Alexiou M, Agulnik SI, Gibson-Brown JJ, Cebra-Thomas J, Bollag RJ, Silver LM, Papaioannou VE. Expression of the T-box family genes, Tbx1–Tbx5, during early mouse development. Dev. Dyn. 1996;206:379–390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

