Sustained stimulation shifts the mechanism of endocytosis from dynamin-1-dependent rapid endocytosis to clathrin- and dynamin-2-mediated slow endocytosis in chromaffin cells
- PMID: 11959911
- PMCID: PMC122953
- DOI: 10.1073/pnas.082658499
Sustained stimulation shifts the mechanism of endocytosis from dynamin-1-dependent rapid endocytosis to clathrin- and dynamin-2-mediated slow endocytosis in chromaffin cells
Erratum in
- Proc Natl Acad Sci U S A 2002 Jun 25;99(13):9082
Abstract
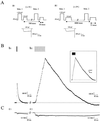
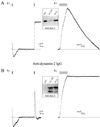
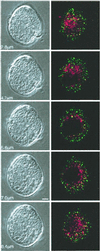
Transient stimulation of secretion in calf chromaffin cells is invariably followed by rapid endocytosis (RE), a clathrin- and K(+)-independent process with a half time of several seconds. Here we show that when exocytosis is triggered in a more sustained manner, a much slower form of endocytosis (SE) replaces RE. SE is complete within 10 min and is abolished when anticlathrin antibodies are introduced into the cell or when intracellular K(+) is removed. RE, but not SE, is blocked by intracellular administration of antidynamin-1 antibodies; the inverse specificity was found for antidynamin-2 antibodies. Replacement of extracellular Ca(2+) by Ba(2+) or Sr(2+) completely blocked RE but had little effect on SE. Thus chromaffin cells exhibit two kinetically and mechanistically distinct forms of endocytosis that are coupled to different extents of exocytosis and are mediated by different isoforms of dynamin. We surmise that RE is associated with the transient fusion ("kiss-and-run") mechanism of transmitter release and is the prevalent means of vesicle recapture and recycling under normal physiological conditions, whereas the clathrin-based SE mechanism comes into play only at higher levels of stimulation and may be associated with complete fusion of vesicles with the plasma membrane.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

