Supramolecular recognition: on the kinetic lability of thermodynamically stable host-guest association complexes
- PMID: 11959933
- PMCID: PMC122677
- DOI: 10.1073/pnas.052587499
Supramolecular recognition: on the kinetic lability of thermodynamically stable host-guest association complexes
Abstract
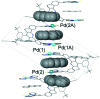
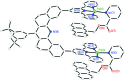
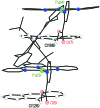
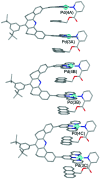
A molecular receptor consisting of a spacer bearing two cofacially disposed terpyridyl-palladium-ligand (terpy-Pd-L) units rigidly separated by about 7 A has been investigated for molecular recognition of planar aromatic molecules. It is found that although the receptor forms stable 1:2 host-guest association complexes with 9-methylanthracene (9-MA), the guest undergoes very rapid site exchange within the receptor and with external free 9-MA. A crystal structure of the 2:1 adduct shows one 9-MA in the molecular cleft defined by the two terpy-Pd-L units and the other resides on an outside face of one terpy-Pd-L unit. To establish the site residency time of the guests, a number of tethered molecules were prepared. These involve an anthracene molecule tethered to a pyridine ligand bound to the palladium atoms to form intramolecular host-guest adducts. Rotating-frame Overhauser effects were used to infer the site residency of the anthracene guests in the receptor. Variable-temperature (1)H NMR spectroscopy of the intramolecular host-guest complexes has revealed that the site residency time of the anthracene guests is 1.6 x 10(-5) sec at 20 degrees C and 1.3 sec at -90 degrees C in acetone solution. Whereas the guests are thermodynamically stable, they are kinetically very labile. A crystal structure of one of the tethered host-guest adducts reveals the expected structure which is the same as that determined in solution by (1)H rotating-frame Overhauser enhancement spectroscopy experiments.
Figures
References
-
- Goshe A J, Crowley J D, Bosnich B. Helv Chim Acta. 2001;84:2971–2985.
-
- Leininger S, Olenyuk B, Stang P J. Chem Rev. 2000;100:853–908. - PubMed
-
- Swiegers G F, Malefetse T J. Chem Rev. 2000;100:3483–3537. - PubMed
-
- Jones C J. Chem Soc Rev. 1998;27:289–299.
-
- Biradha K, Fujita M. In: Advances in Supramolecular Chemistry. Gokel G W, editor. Stamford, CT: JAI Press; 2000. pp. 1–39.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

