Selective immobilization of proteins to self-assembled monolayers presenting active site-directed capture ligands
- PMID: 11959956
- PMCID: PMC122719
- DOI: 10.1073/pnas.072685299
Selective immobilization of proteins to self-assembled monolayers presenting active site-directed capture ligands
Abstract
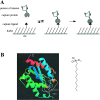
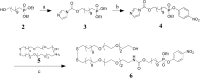
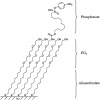
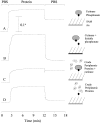
This paper describes a method for the selective and covalent immobilization of proteins to surfaces with control over the density and orientation of the protein. The strategy is based on binding of the serine esterase cutinase to a self-assembled monolayer presenting a phosphonate ligand and the subsequent displacement reaction that covalently binds the ligand to the enzyme active site. Surface plasmon resonance (SPR) spectroscopy showed that cutinase binds irreversibly to a monolayer presenting the capture ligand at a density of 1% mixed among tri(ethylene glycol) groups. The covalent immobilization is specific for cutinase, and the glycol-terminated monolayer effectively prevents unwanted nonspecific adsorption of proteins. To demonstrate that the method could be used to immobilize proteins of interest, a cutinase-calmodulin fusion protein was constructed and immobilized to the monolayer. SPR showed that calcineurin selectively associated with the immobilized calmodulin. This capture ligand immobilization method combines the advantages that the immobilization reaction is highly selective for the intended protein, the tether is covalent and, hence, stable, and the method avoids the need for synthetic modification and rigorous purification of proteins before immobilization. These characteristics make the method well suited to a range of applications and, in particular, for constructing protein microarrays.
Figures

References
-
- Fields S. Science. 2001;291:1221–1224. - PubMed
-
- Zhu H, Snyder M. Curr Opin Chem Biol. 2001;5:40–45. - PubMed
-
- MacBeath G. Nat Biotechnol. 2001;19:828–829. - PubMed
-
- MacBeath G, Schreiber S L. Science. 2000;289:1760–1763. - PubMed
-
- Zhu H, Bilgin M, Bangham R, Hall D, Casamayer A, Bertone P, Lan N, Jansen R, Bidlingmaier S, Houfek T. Science. 2001;293:2101–2105. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

