Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension
- PMID: 11959962
- PMCID: PMC122735
- DOI: 10.1073/pnas.072650799
Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension
Abstract
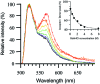
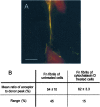
Evidence is emerging that mechanical stretching can alter the functional states of proteins. Fibronectin (Fn) is a large, extracellular matrix protein that is assembled by cells into elastic fibrils and subjected to contractile forces. Assembly into fibrils coincides with expression of biological recognition sites that are buried in Fn's soluble state. To investigate how supramolecular assembly of Fn into fibrillar matrix enables cells to mechanically regulate its structure, we used fluorescence resonance energy transfer (FRET) as an indicator of Fn conformation in the fibrillar matrix of NIH 3T3 fibroblasts. Fn was randomly labeled on amine residues with donor fluorophores and site-specifically labeled on cysteine residues in modules FnIII(7) and FnIII(15) with acceptor fluorophores. Intramolecular FRET was correlated with known structural changes of Fn in denaturing solution, then applied in cell culture as an indicator of Fn conformation within the matrix fibrils of NIH 3T3 fibroblasts. Based on the level of FRET, Fn in many fibrils was stretched by cells so that its dimer arms were extended and at least one FnIII module unfolded. When cytoskeletal tension was disrupted using cytochalasin D, FRET increased, indicating refolding of Fn within fibrils. These results suggest that cell-generated force is required to maintain Fn in partially unfolded conformations. The results support a model of Fn fibril elasticity based on unraveling and refolding of FnIII modules. We also observed variation of FRET between and along single fibrils, indicating variation in the degree of unfolding of Fn in fibrils. Molecular mechanisms by which mechanical force can alter the structure of Fn, converting tensile forces into biochemical cues, are discussed.
Figures

References
-
- Sechler J L, Schwarzbauer J E. J Biol Chem. 1998;273:25533–25536. - PubMed
-
- Sechler J L, Corbett S A, Wenk M B, Schwarzbauer J E. Ann NY Acad Sci. 1998;857:143–154. - PubMed
-
- Darribere T, Schwarzbauer J E. Mech Dev. 2000;92:239–250. - PubMed
-
- Leahy D J, Aukhil I, Erickson H P. Cell. 1996;84:155–164. - PubMed
-
- Spitzfaden C, Grant R P, Mardon H J, Campbell I D. J Mol Biol. 1997;265:565–579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

