Investigation of the role of ansocalcin in the biomineralization in goose eggshell matrix
- PMID: 11959964
- PMCID: PMC122738
- DOI: 10.1073/pnas.072658899
Investigation of the role of ansocalcin in the biomineralization in goose eggshell matrix
Abstract
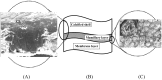
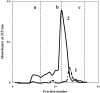
The role of proteins in biomineralization and the mechanism of eggshell formation are not well understood. We have isolated and purified the major protein, ansocalcin from goose eggshell matrix. The amino acid sequence study indicates that ansocalcin is homologous to the chicken eggshell protein, ovocleidin 17, and C-type lectins. Ansocalcin nucleates polycrystalline aggregates of calcite crystals in in vitro mineralization experiments. The polycrystalline aggregates obtained at higher concentration of ansocalcin appears to be similar to the crystals observed at the mamillary layer of the eggshell.
Figures


References
-
- Weiner S. Biochemistry. 1983;22:4139–4145.
-
- Berman A, Addadi L, Kvick A, Leiserowitz L, Nelson M, Weiner S. Science. 1990;250:664. - PubMed
-
- Mann S. Struct Bonding (Berlin) 1983;54:125–174.
-
- Lowenstam H A. Science. 1981;211:1126–1131. - PubMed
-
- Soten I, Ozin G A. Curr Opin Colloid Interface Sci. 1999;4:325–337.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

