Structural basis for recruitment of CBP/p300 by hypoxia-inducible factor-1 alpha
- PMID: 11959990
- PMCID: PMC122775
- DOI: 10.1073/pnas.082117899
Structural basis for recruitment of CBP/p300 by hypoxia-inducible factor-1 alpha
Abstract
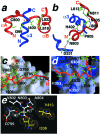
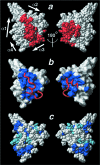
Adaptation to hypoxia is mediated by transactivation of hypoxia-responsive genes by hypoxia-inducible factor-1 (HIF-1) in complex with the CBP and p300 transcriptional coactivators. We report the solution structure of the cysteine/histidine-rich 1 (CH1) domain of p300 bound to the C-terminal transactivation domain of HIF-1 alpha. CH1 has a triangular geometry composed of four alpha-helices with three intervening Zn(2+)-coordinating centers. CH1 serves as a scaffold for folding of the HIF-1 alpha C-terminal transactivation domain, which forms a vise-like clamp on the CH1 domain that is stabilized by extensive hydrophobic and polar interactions. The structure reveals the mechanism of specific recognition of p300 by HIF-1 alpha, and shows how HIF-1 alpha transactivation is regulated by asparagine hydroxylation.
Figures

References
-
- Semenza G L. Annu Rev Cell Dev Biol. 1999;15:551–578. - PubMed
-
- Semenza G L. Trends Mol Med. 2001;7:345–350. - PubMed
-
- Salceda S, Caro J. J Biol Chem. 1997;272:22642–22647. - PubMed
-
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara J M, Lane W S, Kaelin W G., Jr Science. 2001;292:464–468. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

