IRAK-4: a novel member of the IRAK family with the properties of an IRAK-kinase
- PMID: 11960013
- PMCID: PMC122810
- DOI: 10.1073/pnas.082100399
IRAK-4: a novel member of the IRAK family with the properties of an IRAK-kinase
Abstract
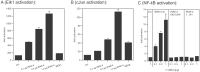
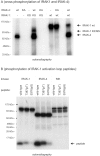
Toll/IL-1 receptor family members are central components of host defense mechanisms in a variety of species. One well conserved element in their signal transduction is Ser/Thr kinases, which couple early signaling events in a receptor complex at the plasma membrane to larger signalosomes in the cytosol. The fruit fly Drosophila melanogaster has one member of this family of kinases, termed Pelle. The complexity of this pathway is vastly increased in vertebrates, and several Pelle homologs have been described and termed IL-1 receptor-associated kinase (IRAK). Here we report the identification of a novel and distinct member of the IRAK family, IRAK-4. IRAK-4 is the closest human homolog to Pelle. Endogenous IRAK-4 interacts with IRAK-1 and TRAF6 in an IL-1-dependent manner, and overexpression of IRAK-4 can activate NF-kappa B as well as mitogen-activated protein (MAP) kinase pathways. Most strikingly, and in contrast to the other IRAKs, IRAK-4 depends on its kinase activity to activate NF-kappa B. In addition, IRAK-4 is able to phosphorylate IRAK-1, and overexpression of dominant-negative IRAK-4 is blocking the IL-1-induced activation and modification of IRAK-1, suggesting a role of IRAK-4 as a central element in the early signal transduction of Toll/IL-1 receptors, upstream of IRAK-1.
Figures



References
-
- Kollewe, C. & Martin, M. U. (2002) Signal Trans., in press.
-
- Wesche, H. & Martin, M. U. (2002) Biochim. Biophys. Acta, in press. - PubMed
-
- Dinarello C A. Blood. 1996;87:2095–2147. - PubMed
-
- Fallon P G, Allen R L, Rich T. Trends Immunol. 2001;22:63–66. - PubMed
-
- Ulevitch R J, Tobias P S. Curr Opin Immunol. 1999;11:19–22. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

