Amyloid fibers are water-filled nanotubes
- PMID: 11960014
- PMCID: PMC122814
- DOI: 10.1073/pnas.042681399
Amyloid fibers are water-filled nanotubes
Abstract
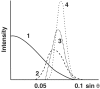
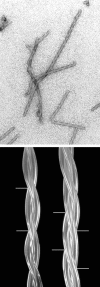
A study of papers on amyloid fibers suggested to us that cylindrical beta-sheets are the only structures consistent with some of the x-ray and electron microscope data. We then found that our own 7-year-old and hitherto enigmatic x-ray diagram of poly-L-glutamine fits a cylindrical sheet of 31 A diameter made of beta-strands with 20 residues per helical turn. Successive turns are linked by hydrogen bonds between both the main chain and side chain amides, and side chains point alternately into and out of the cylinder. Fibers of the exon-1 peptide of huntingtin and of the glutamine- and asparagine-rich region of the yeast prion Sup35 give the same underlying x-ray diagrams, which show that they have the same structure. Electron micrographs show that the 100-A-thick fibers of the Sup35 peptide are ropes made of three protofibrils a little over 30 A thick. They have a measured mass of 1,450 Da/A, compared with 1,426 Da/A for a calculated mass of three protofibrils each with 20 residues per helical turn wound around each other with a helical pitch of 510 A. Published x-ray diagrams and electron micrographs show that fibers of synuclein, the protein that forms the aggregates of Parkinson disease, consist of single cylindrical beta-sheets. Fibers of Alzheimer A beta fragments and variants are probably made of either two or three concentric cylindrical beta-sheets. Our structure of poly-L-glutamine fibers may explain why, in all but one of the neurodegenerative diseases resulting from extension of glutamine repeats, disease occurs when the number of repeats exceeds 37-40. A single helical turn with 20 residues would be unstable, because there is nothing to hold it in place, but two turns with 40 residues are stabilized by the hydrogen bonds between their amides and can act as nuclei for further helical growth. The A beta peptide of Alzheimer's disease contains 42 residues, the best number for nucleating further growth. All these structures are very stable; the best hope for therapies lies in preventing their growth.
Figures



References
-
- Perutz M. Protein Structure: New Approaches to Disease & Therapy. New York: Freeman; 1992.
-
- Perutz M F. Trans Faraday Soc. 1946;42B:187–195.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

