Supercool or dehydrate? An experimental analysis of overwintering strategies in small permeable arctic invertebrates
- PMID: 11960026
- PMCID: PMC122837
- DOI: 10.1073/pnas.082580699
Supercool or dehydrate? An experimental analysis of overwintering strategies in small permeable arctic invertebrates
Abstract
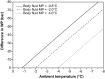
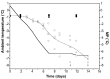
Soil invertebrate survival in freezing temperatures has generally been considered in the light of the physiological adaptations seen in surface living insects. These adaptations, notably the ability to supercool, have evolved in concert with surface invertebrates' ability to retain body water in a dry environment. However, most soil invertebrates are orders of magnitude less resistant to desiccation than these truly terrestrial insects, opening the possibility that the mechanisms involved in their cold-hardiness are also of a radically different nature. Permeable soil invertebrates dehydrate when exposed in frozen soil. This dehydration occurs because the water vapor pressure of supercooled water is higher than that of ice at the same temperature. The force of this vapor pressure difference is so large that even a few degrees of supercooling will result in substantial water loss, continuing until the vapor pressure of body fluids equals that of the surrounding ice. At this stage, the risk of tissue ice formation has been eliminated, and subzero survival is ensured. Here we show that these soil invertebrates do not base their winter survival on supercooling, as do many other ectotherms, but instead dehydrate and equilibrate their body-fluid melting point to the ambient temperature. They can achieve this equilibration even at the extreme cooling rates seen in polar soils.
Figures


References
-
- Zachariassen K E. Physiol Rev. 1985;65:799–831. - PubMed
-
- Block W. Philos Trans R Soc London B. 1990;326:613–633.
-
- Lee R E. In: Insects at Low Temperature. Lee R E, Denlinger D, editors. New York: Chapman & Hall; 1991. pp. 17–46.
-
- Pickup J. Funct Ecol. 1990;4:257–264.
-
- Wharton D, Block W. Funct Ecol. 1993;7:578–584.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

