DNA cleavage and packaging proteins encoded by genes U(L)28, U(L)15, and U(L)33 of herpes simplex virus type 1 form a complex in infected cells
- PMID: 11967295
- PMCID: PMC136146
- DOI: 10.1128/jvi.76.10.4785-4791.2002
DNA cleavage and packaging proteins encoded by genes U(L)28, U(L)15, and U(L)33 of herpes simplex virus type 1 form a complex in infected cells
Abstract
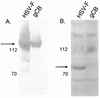
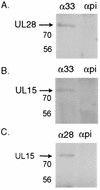
Previous studies have indicated that the U(L)6, U(L)15, U(L)17, U(L)28, U(L)32, and U(L)33 genes are required for the cleavage and packaging of herpes simplex viral DNA. To identify proteins that interact with the U(L)28-encoded DNA binding protein of herpes simplex virus type 1 (HSV-1), a previously undescribed rabbit polyclonal antibody directed against the U(L)28 protein fused to glutathione S-transferase was used to immunopurify U(L)28 and the proteins with which it associated. It was found that the antibody specifically coimmunoprecipitated proteins encoded by the genes U(L)28, U(L)15, and U(L)33 from lysates of both HEp-2 cells infected with HSV-1(F) and insect cells infected with recombinant baculoviruses expressing these three proteins. In reciprocal reactions, antibodies directed against the U(L)15- or U(L)33-encoded proteins also coimmunoprecipitated the U(L)28 protein. The coimmunoprecipitation of the three proteins from HSV-infected cells confirms earlier reports of an association between the U(L)28 and U(L)15 proteins and represents the first evidence of the involvement of the U(L)33 protein in this complex.
Figures



References
-
- Abbotts, A. P., V. G. Preston, M. Hughes, A. H. Patel, and N. D. Stow. 2000. Interaction of the herpes simplex virus type 1 packaging protein UL15 with full-length and deleted forms of the UL28 protein. J. Gen. Virol. 81:2999-3009. - PubMed
-
- Addison, C., F. J. Rixon, J. W. Palfreyman, M. O'Hara, and V. G. Preston. 1984. Characterisation of a herpes simplex virus type 1 mutant which has a temperature-sensitive defect in penetration of cells and assembly of capsids. Virology 138:246-259. - PubMed
-
- Addison, C., F. J. Rixon, and V. G. Preston. 1990. Herpes simplex virus type 1 UL28 gene product is important for the formation of mature capsids. J. Gen. Virol. 71:2377-2384. - PubMed
-
- al-Kobaisi, M. F., F. J. Rixon, I. McDougall, and V. G. Preston. 1991. The herpes simplex virus UL33 gene product is required for the assembly of full capsids. Virology 180:380-388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

