Ebola virus VP40 drives the formation of virus-like filamentous particles along with GP
- PMID: 11967302
- PMCID: PMC136157
- DOI: 10.1128/jvi.76.10.4855-4865.2002
Ebola virus VP40 drives the formation of virus-like filamentous particles along with GP
Abstract
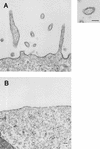
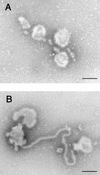
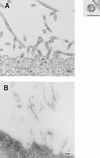
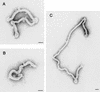
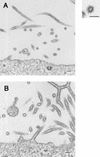
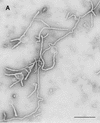
Using biochemical assays, it has been demonstrated that expression of Ebola virus VP40 alone in mammalian cells induced production of particles with a density similar to that of virions. To determine the morphological properties of these particles, cells expressing VP40 and the particles released from the cells were examined by electron microscopy. VP40 induced budding from the plasma membrane of filamentous particles, which differed in length but had uniform diameters of approximately 65 nm. When the Ebola virus glycoprotein (GP) responsible for receptor binding and membrane fusion was expressed in cells, we found pleomorphic particles budding from the plasma membrane. By contrast, when GP was coexpressed with VP40, GP was found on the filamentous particles induced by VP40. These results demonstrated the central role of VP40 in formation of the filamentous structure of Ebola virions and may suggest an interaction between VP40 and GP in morphogenesis.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

