Inhibition of histone deacetylases induces bovine leukemia virus expression in vitro and in vivo
- PMID: 11967319
- PMCID: PMC136152
- DOI: 10.1128/jvi.76.10.5034-5042.2002
Inhibition of histone deacetylases induces bovine leukemia virus expression in vitro and in vivo
Abstract
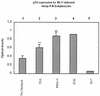
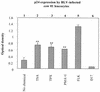
Packaging into nucleosomes results in a global transcriptional repression as a consequence of exclusion of sequence-specific factors. This inhibition can be relieved by using inhibitors of histone deacetylases, acetylation being a major characteristic of transcriptionally active chromatin. Paradoxically, the expression of only approximately 2% of the total cellular genes is modulated by histone hyperacetylation. To unravel the potential role of this transcriptional control on BLV expression, we tested the effect of two highly specific inhibitors of deacetylases, trichostatin A (TSA) and trapoxin (TPX). Our results demonstrate that treatment with TSA efficiently enhanced long terminal repeat-directed gene expression of integrated reporter constructs in heterologous D17 stable cell lines. To further examine the biological relevance of these observations made in vitro, we analyzed ex vivo-isolated peripheral blood mononuclear cells (PBMCs) from bovine leukemia virus (BLV)-infected sheep. TSA deacetylase inhibitor induced a drastic increase in viral expression at levels comparable to those induced by treatment with phorbol-12-myristate 13-acetate and ionomycin, the most efficient activators of BLV expression known to date. TSA acted directly on BLV-infected B lymphocytes to increase viral expression and does not seem to require T-cell cooperation. Inhibition of deacetylation after treatment with TSA or TPX also significantly increased viral expression in PBMCs from cattle, the natural host for BLV. Together, our results show that BLV gene expression is, like that of a very small fraction of cellular genes, also regulated by deacetylation.
Figures




Similar articles
-
Overlapping CRE and E box motifs in the enhancer sequences of the bovine leukemia virus 5' long terminal repeat are critical for basal and acetylation-dependent transcriptional activity of the viral promoter: implications for viral latency.J Virol. 2004 Dec;78(24):13848-64. doi: 10.1128/JVI.78.24.13848-13864.2004. J Virol. 2004. PMID: 15564493 Free PMC article.
-
Transcriptional activation and chromatin remodeling of the HIV-1 promoter in response to histone acetylation.EMBO J. 1996 Mar 1;15(5):1112-20. EMBO J. 1996. PMID: 8605881 Free PMC article.
-
Peripheral blood mononuclear cells from sheep infected with a variant of bovine leukemia virus synthesize envelope glycoproteins but fail to induce syncytia in culture.J Virol. 1996 Sep;70(9):6296-303. doi: 10.1128/JVI.70.9.6296-6303.1996. J Virol. 1996. PMID: 8709257 Free PMC article.
-
Bovine leukemia virus-induced persistent lymphocytosis in cattle does not correlate with increased ex vivo survival of B lymphocytes.J Virol. 1999 Feb;73(2):1127-37. doi: 10.1128/JVI.73.2.1127-1137.1999. J Virol. 1999. PMID: 9882314 Free PMC article.
-
Trichostatin A and trapoxin: novel chemical probes for the role of histone acetylation in chromatin structure and function.Bioessays. 1995 May;17(5):423-30. doi: 10.1002/bies.950170510. Bioessays. 1995. PMID: 7786288 Review.
Cited by
-
Mechanisms of leukemogenesis induced by bovine leukemia virus: prospects for novel anti-retroviral therapies in human.Retrovirology. 2007 Mar 16;4:18. doi: 10.1186/1742-4690-4-18. Retrovirology. 2007. PMID: 17362524 Free PMC article. Review.
-
Characterization of new RNA polymerase III and RNA polymerase II transcriptional promoters in the Bovine Leukemia Virus genome.Sci Rep. 2016 Aug 22;6:31125. doi: 10.1038/srep31125. Sci Rep. 2016. PMID: 27545598 Free PMC article.
-
Comparison of bovine leukemia virus (BLV) and CMV promoter-driven reporter gene expression in BLV-infected and non-infected cells.Genet Vaccines Ther. 2004 Aug 24;2(1):11. doi: 10.1186/1479-0556-2-11. Genet Vaccines Ther. 2004. PMID: 15327692 Free PMC article.
-
Reduced cell turnover in bovine leukemia virus-infected, persistently lymphocytotic cattle.J Virol. 2003 Dec;77(24):13073-83. doi: 10.1128/jvi.77.24.13073-13083.2003. J Virol. 2003. PMID: 14645564 Free PMC article.
-
Chemoresistance to Valproate Treatment of Bovine Leukemia Virus-Infected Sheep; Identification of Improved HDAC Inhibitors.Pathogens. 2012 Oct 8;1(2):65-82. doi: 10.3390/pathogens1020065. Pathogens. 2012. PMID: 25436765 Free PMC article.
References
-
- Adcock, I. M. 2001. Glucocorticoid-regulated transcription factors. Pulm. Pharmacol. Ther. 14:211-219. - PubMed
-
- Ayer, D. E., Q. A. Lawrence, and R. N. Eisenman. 1995. Mad-Max transcriptional repression is mediated by ternary complex formation with mammalian homologs of yeast repressor Sin3. Cell 80:767-776. - PubMed
-
- Baliga, V., and J. F. Ferrer. 1977. Expression of the bovine leukemia virus and its internal antigen in blood lymphocytes. Proc. Soc. Exp. Biol. Med. 156:388-391. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

