Structure of the transition state of gating in the acetylcholine receptor channel pore: a phi-value analysis
- PMID: 11969415
- PMCID: PMC6442467
- DOI: 10.1021/bi011864f
Structure of the transition state of gating in the acetylcholine receptor channel pore: a phi-value analysis
Abstract
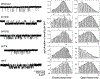
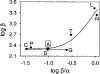
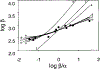
The gating mechanism of the acetylcholine receptor channel (AChR) was investigated by using rate equilibrium linear free energy relationships (LFERs) to probe the transition state between the closed and open conformations. The properties of the transition state of gating in the second transmembrane segment (M2) of the delta subunit, one of the five homologous pore-lining segments, was measured on a residue-by-residue basis. Series of point mutations were engineered at individual positions of this domain, and the corresponding constructs were characterized electrophysiologically, at the single-channel level. Fully liganded AChR opening and closing rate constants were estimated, and Phi-values (which are a measure of the extent of the conformational change realized at the transition state) were calculated for each reaction series as the slope of the Brønsted relationship (log rate constant versus log equilibrium constant). Our results indicate that, at the transition state of gating, the extracellular half of deltaM2 partly resembles the open state (Phi-values between 0.24 and 0.38) while the intracellular half completely resembles the closed state (Phi-values between -0.18 and 0.03), with a break point near the middle of the M2 segment. This suggests that during gating the two halves of deltaM2 move asynchronously, with the rearrangement of the extracellular portion preceding (following) that of the intracellular part of deltaM2 during opening (closing). This particular sequence of molecular events indicates that the gating conformational change, which starts at the extracellular acetylcholine-binding sites (when opening), does not propagate exclusively along the primary sequence of the protein. In addition, our data are consistent with the deltaM2 segment bending or swiveling around its central residues during gating. We also elaborate on unsettled aspects of the analysis such as the accuracy of two-point LFERs, the physical interpretation of fractional Phi-values, and the existence of single versus parallel transition states for the gating reaction.
Figures




References
-
- Imoto K, Methfessel C, Sakmann B, Mishina M, Mori Y, Konno T, Fukuda K, Kurasaki M, Bujo H, and Fujita Y (1986) Nature 324, 670–674. - PubMed
-
- Imoto K, Busch C, Sakmann B, Mishina M, Konno T,Nakai J, Bujo H, Mori Y, Fukuda K, and Numa S (1988) Nature 335, 645–648. - PubMed
-
- White BH, and Cohen JB (1992) J. Biol. Chem 267, 15770–15783. - PubMed
-
- Akabas MH, Kaufmann C, Archdeacon P, and Karlin A (1994) Neuron 13, 919–927. - PubMed
-
- Zhang H, and Karlin A (1998) Biochemistry 37, 7952–7964. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

