Rab2 GTPase regulates vesicle trafficking between the endoplasmic reticulum and the Golgi bodies and is important to pollen tube growth
- PMID: 11971147
- PMCID: PMC150694
- DOI: 10.1105/tpc.000836
Rab2 GTPase regulates vesicle trafficking between the endoplasmic reticulum and the Golgi bodies and is important to pollen tube growth
Abstract
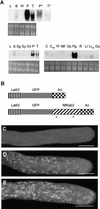
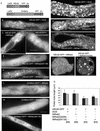
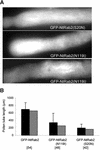
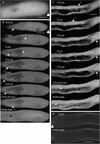
Pollen tube elongation depends on the secretion of large amounts of membrane and cell wall materials at the pollen tube tip to sustain rapid growth. A large family of RAS-related small GTPases, Rabs or Ypts, is known to regulate both anterograde and retrograde trafficking of transport vesicles between different endomembrane compartments and the plasma membrane in mammalian and yeast cells. Studies on the functional roles of analogous plant proteins are emerging. We report here that a tobacco pollen-predominant Rab2, NtRab2, functions in the secretory pathway between the endoplasmic reticulum and the Golgi in elongating pollen tubes. Green fluorescent protein-NtRab2 fusion protein localized to the Golgi bodies in elongating pollen tubes. Dominant-negative mutations in NtRab2 proteins inhibited their Golgi localization, blocked the delivery of Golgi-resident as well as plasmalemma and secreted proteins to their normal locations, and inhibited pollen tube growth. On the other hand, when green fluorescent protein-NtRab2 was over-expressed in transiently transformed leaf protoplasts and epidermal cells, in which NtRab2 mRNA have not been observed to accumulate to detectable levels, these proteins did not target efficiently to Golgi bodies. Together, these observations indicate that NtRab2 is important for trafficking between the endoplasmic reticulum and the Golgi bodies in pollen tubes and may be specialized to optimally support the high secretory demands in these tip growth cells.
Figures

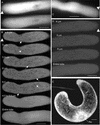

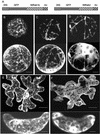
References
-
- Allan, B.B., Moyer, B.D., and Balch, W.E. (2000). Rab1 recruitment of p115 into a cis-SNARE complex: Programming budding COPII vesicles for fusion. Science 289, 444–447. - PubMed
-
- Andreeva, A.V., Zheng, H., Saint-Jore, C.M., Kutuzov, M.A., Evans, D.E., and Hawes, C.R. (2000). Organization of transport from endoplasmic reticulum to Golgi in higher plants. Biochem. Soc. Trans. 28, 505–512. - PubMed
-
- Arabidopsis Genome Initiative. (2000). Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408, 796–815. - PubMed
-
- Ayala, J., Olofsson, B., Touchot, N., Zahraoui, A., Tavitian, A., and Prochiantz, A. (1989). Developmental and regional expression of three new members of the ras-gene family in the mouse brain. J. Neurosci. Res. 22, 384–389. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

