Time-resolved resonance Raman analysis of chromophore structural changes in the formation and decay of rhodopsin's BSI intermediate
- PMID: 11971736
- PMCID: PMC1440918
- DOI: 10.1021/ja012666e
Time-resolved resonance Raman analysis of chromophore structural changes in the formation and decay of rhodopsin's BSI intermediate
Abstract
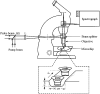
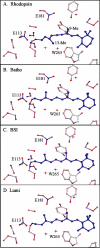
Time-resolved resonance Raman microchip flow experiments are performed to obtain the vibrational spectrum of the chromophore in rhodopsin's BSI intermediate and to probe structural changes in the bathorhodopsin-to-BSI and BSI-to-lumirhodopsin transitions. Kinetic Raman spectra from 250 ns to 3 micros identify the key vibrational features of BSI. BSI exhibits relatively intense HOOP modes at 886 and 945 cm(-1) that are assigned to C(14)H and C(11)H=C(12)H A(u) wags, respectively. This result suggests that in the bathorhodopsin-to-BSI transition the highly strained all-trans chromophore has relaxed in the C(10)-C(11)=C(12)-C(13) region, but is still distorted near C(14). The low frequency of the 11,12 A(u) HOOP mode in BSI compared with that of lumirhodopsin and metarhodopsin I indicates weaker coupling between the 11H and 12H wags due to residual distortion of the BSI chromophore near C(11)=C(12). The C=NH(+) stretching mode in BSI at 1653 cm(-1) exhibits a normal deuteriation induced downshift of 23 cm(-1), implying that there is no significant structural rearrangement of the Schiff base counterion region in the transition of bathorhodopsin to BSI. However, a dramatic Schiff base environment change occurs in the BSI-to-lumirhodopsin transition, because the 1638 cm(-1) C=NH(+) stretching mode in lumirhodopsin is unusually low and shifts only 7 cm(-1) in D(2)O, suggesting that it has essentially no H-bonding acceptor. With these data we can for the first time compare and discuss the room temperature resonance Raman vibrational structure of all the key intermediates in visual excitation.
Figures






References
-
- Mathies RA, Lugtenburg J. Handbook of Biological Physics. In: Stavenga DG, deGrip WJ, Pugh EN Jr., editors. Vol. 3. Elsevier Science Press; Amsterdam, The Netherlands: 2000. pp. 55–90.
-
- Schoenlein RW, Peteanu LA, Mathies RA, Shank CV. Science. 1991;254:412–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

