Antimicrobial use and antimicrobial resistance: a population perspective
- PMID: 11971765
- PMCID: PMC2730242
- DOI: 10.3201/eid0804.010312
Antimicrobial use and antimicrobial resistance: a population perspective
Erratum in
- Emerg Infect Dis 2002 May;8(5):540
Abstract
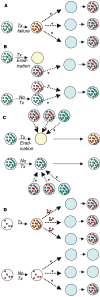
The need to stem the growing problem of antimicrobial resistance has prompted multiple, sometimes conflicting, calls for changes in the use of antimicrobial agents. One source of disagreement concerns the major mechanisms by which antibiotics select resistant strains. For infections like tuberculosis, in which resistance can emerge in treated hosts through mutation, prevention of antimicrobial resistance in individual hosts is a primary method of preventing the spread of resistant organisms in the community. By contrast, for many other important resistant pathogens, such as penicillin-resistant Streptococcus pneumoniae, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant Enterococcus faecium resistance is mediated by the acquisition of genes or gene fragments by horizontal transfer; resistance in the treated host is a relatively rare event. For these organisms, indirect, population-level mechanisms of selection account for the increase in the prevalence of resistance. These mechanisms can operate even when treatment has a modest, or even negative, effect on an individual host's colonization with resistant organisms.
Figures
References
-
- Boyce JM. Treatment and control of colonization in the prevention of nosocomial infections. Infect Control Hosp Epidemiol. 1996;17:256–61. - PubMed
-
- Brun-Buisson C, Legrand P, Rauss A, Richard C, Montravers F, Besbes M, et al. Intestinal decontamination for control of nosocomial multiresistant gram-negative bacilli. Study of an outbreak in an intensive care unit. [Medline]. Ann Intern Med. 1989;110:873–81. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
