Requirement of Ca2+ and CaMKII for Stat1 Ser-727 phosphorylation in response to IFN-gamma
- PMID: 11972023
- PMCID: PMC122886
- DOI: 10.1073/pnas.052159099
Requirement of Ca2+ and CaMKII for Stat1 Ser-727 phosphorylation in response to IFN-gamma
Abstract
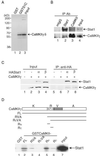
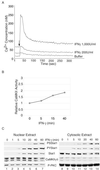
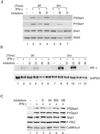
In response to IFN-gamma, the latent cytoplasmic protein signal transducers and activators of transcription 1 (Stat1) becomes phosphorylated on Y701, dimerizes, and accumulates in the nucleus to activate transcription of IFN-gamma-responsive genes. For maximal gene activation, S727 in the transcription activation domain of Stat1 also is inducibly phosphorylated by IFN-gamma. We previously purified a group of nuclear proteins that interact specifically with the Stat1 transcription activation domain. In this report, we identified one of them as the multifunctional Ca(2+)/calmodulin-dependent kinase (CaMK) II. We demonstrate that IFN-gamma mobilizes a Ca(2+) flux in cells and activates CaMKII. CaMKII can interact directly with Stat1 and phosphorylate Stat1 on S727 in vitro. Inhibition of Ca(2+) flux or CaMKII results in a lack of S727 phosphorylation and Stat1-dependent gene activation, suggesting in vivo phosphorylation of Stat1 S727 by CaMKII. Thus two different cellular signaling events, IFN-gamma receptor occupation and Ca(2+) flux, are required for Stat1 to achieve maximal transcriptional activation through regulation of phosphorylation.
Figures


References
-
- Darnell J E., Jr Science. 1997;277:1630–1635. - PubMed
-
- Leonard W J, O'Shea J J. Annu Rev Immunol. 1998;16:293–322. - PubMed
-
- Becker S, Groner B, Muller C W. Nature (London) 1998;394:145–151. - PubMed
-
- Chen X, Vinkemeier U, Zhao Y, Jeruzalmi D, Darnell J E, Jr, Kuriyan J. Cell. 1998;93:827–839. - PubMed
-
- Bhattacharya S, Eckner R, Grossman S, Oldread E, Arany Z, D'Andrea A, Livingston D M. Nature (London) 1996;383:344–347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

