Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene Scn5a
- PMID: 11972032
- PMCID: PMC122928
- DOI: 10.1073/pnas.082121299
Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene Scn5a
Abstract
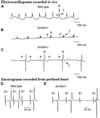
Voltage-gated sodium channels drive the initial depolarization phase of the cardiac action potential and therefore critically determine conduction of excitation through the heart. In patients, deletions or loss-of-function mutations of the cardiac sodium channel gene, SCN5A, have been associated with a wide range of arrhythmias including bradycardia (heart rate slowing), atrioventricular conduction delay, and ventricular fibrillation. The pathophysiological basis of these clinical conditions is unresolved. Here we show that disruption of the mouse cardiac sodium channel gene, Scn5a, causes intrauterine lethality in homozygotes with severe defects in ventricular morphogenesis whereas heterozygotes show normal survival. Whole-cell patch clamp analyses of isolated ventricular myocytes from adult Scn5a(+/-) mice demonstrate a approximately 50% reduction in sodium conductance. Scn5a(+/-) hearts have several defects including impaired atrioventricular conduction, delayed intramyocardial conduction, increased ventricular refractoriness, and ventricular tachycardia with characteristics of reentrant excitation. These findings reconcile reduced activity of the cardiac sodium channel leading to slowed conduction with several apparently diverse clinical phenotypes, providing a model for the detailed analysis of the pathophysiology of arrhythmias.
Figures



Comment in
-
Unraveling the genetics and mechanisms of cardiac arrhythmia.Proc Natl Acad Sci U S A. 2002 Apr 30;99(9):5755-6. doi: 10.1073/pnas.102171699. Proc Natl Acad Sci U S A. 2002. PMID: 11983875 Free PMC article. Review. No abstract available.
References
-
- Spooner P M, Rosen M R, editors. Foundations of Cardiac Arrhythmias: Basic and Clinical Approaches. New York: Dekker; 2001.
-
- The Sicilian Gambit. Cardiovasc Res. 2001;52:345–360.
-
- Keating M T, Sanguinetti M C. Cell. 2001;104:569–580. - PubMed
-
- Marban E. Nature (London) 2002;415:213–218. - PubMed
-
- Roden D M, Balser J R, George A L, Jr, Anderson M E. Annu Rev Physiol. 2002;64:431–475. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

