Altered sexual and social behaviors in trp2 mutant mice
- PMID: 11972034
- PMCID: PMC122956
- DOI: 10.1073/pnas.082127599
Altered sexual and social behaviors in trp2 mutant mice
Abstract
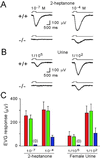
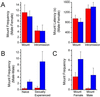
We have used gene targeting to generate mice with a homozygous deficiency in trp2, a cation channel expressed in the vomeronasal organ (VNO). Trp2 mutant animals reveal a striking reduction in the electrophysiological response to pheromones in the VNO, suggesting that trp2 plays a central role in mediating the pheromone response. These mutants therefore afford the opportunity to examine the role of the VNO in the generation of innate sexual and social behaviors in mice. Trp2 mutant males and nursing females are docile and fail to initiate aggressive attacks on intruder males. Male-female sexual behavior appears normal, but trp2 mutant males also vigorously mount other males. These results suggest that the cation channel trp2 is required in the VNO to detect male-specific pheromones that elicit aggressive behaviors and dictate the choice of sexual partners.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

