Association and regulation of casein kinase 2 activity by adenomatous polyposis coli protein
- PMID: 11972058
- PMCID: PMC122884
- DOI: 10.1073/pnas.092143199
Association and regulation of casein kinase 2 activity by adenomatous polyposis coli protein
Abstract
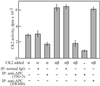
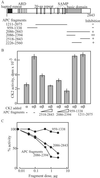
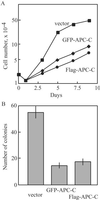
Mutations in the adenomatous polyposis coli (APC) gene are responsible for familial adenomatous polyposis coli and also sporadic colorectal cancer development. By using antibodies raised against the N-terminal region of APC protein, we have detected the variable masses of endogenous APC proteins in individual cell lines established from human colorectal carcinomas caused by nonsense mutations of the gene. Phosphorylation of immunoprecipitates of full-length and truncated APC were observed in in vitro kinase reaction, indicating association of APC with protein kinase activity. The kinase activity complexed with APC was sensitive to heparin and used GTP as phosphoryl donor, suggesting an involvement of casein kinase 2 (CK2). Both CK2alpha- and beta-subunits were found to associate with APC in immunoprecipitates as well as in pull-down assays, with preferential interaction of APC with tetrameric CK2 holoenzyme. In synchronized cell populations, the association of APC with CK2 was cell cycle dependent, with the highest association in G(2)/M. Unexpectedly, APC immunoprecipitates containing full-length APC protein inhibited CK2 in vitro, whereas immunoprecipitates of truncated APC had little effect. This was confirmed by using recombinant APC, and the inhibitory region was localized to the C terminus of APC between residues 2086 and 2394. Overexpression of this fragment in SW480 cells suppressed cell proliferation rates as well as tumorigenesis. These results demonstrate a previously uncharacterized functional interaction between the tumor suppressor protein APC and CK2 and suggest that growth-inhibitory effects of APC may be regulated by inhibition of CK2.
Figures



References
-
- Kinzler K W, Nilnert M C, Su L-K, Vogelstein B, Bryan T, Levy D B, Smith K J, Preisinger A C, Hedge P, McKechnie D, et al. Science. 1991;253:661–664. - PubMed
-
- Fearon E R, Vogelstein B. Cell. 1990;61:759–767. - PubMed
-
- VanEs J H, Giles R H, Clevers H C. Exp Cell Res. 2001;264:126–134. - PubMed
-
- Kishida S, Yamamoto H, Ikeda S, Kishida M, Sakamoto I, Koyama S, Kikuchi A. J Biol Chem. 1998;273:10823–10826. - PubMed
-
- Rubinfeld B, Albers I, Porfiri E, Fiol C, Munemitsu S, Polakis P. Science. 1996;272:1023–1026. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

