Dilated cardiomyopathy in mice deficient for the lysosomal cysteine peptidase cathepsin L
- PMID: 11972068
- PMCID: PMC122932
- DOI: 10.1073/pnas.092637699
Dilated cardiomyopathy in mice deficient for the lysosomal cysteine peptidase cathepsin L
Abstract
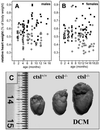
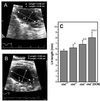
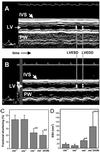
Dilated cardiomyopathy is a frequent cause of heart failure and is associated with high mortality. Progressive remodeling of the myocardium leads to increased dimensions of heart chambers. The role of intracellular proteolysis in the progressive remodeling that underlies dilated cardiomyopathy has not received much attention yet. Here, we report that the lysosomal cysteine peptidase cathepsin L (CTSL) is critical for cardiac morphology and function. One-year-old CTSL-deficient mice show significant ventricular and atrial enlargement that is associated with a comparatively small increase in relative heart weight. Interstitial fibrosis and pleomorphic nuclei were found in the myocardium of the knockout mice. By electron microscopy, CTSL-deficient cardiomyocytes contained multiple large and apparently fused lysosomes characterized by storage of electron-dense heterogeneous material. Accordingly, the assessment of left ventricular function by echocardiography revealed severely impaired myocardial contraction in the CTSL-deficient mice. In addition, echocardiographic and electrocardiographic findings to some degree point to left ventricular hypertrophy that most likely represents an adaptive response to cardiac impairment. The histomorphological and functional alterations of CTSL-deficient hearts result in valve insufficiencies. Furthermore, abnormal heart rhythms, like supraventricular tachycardia, ventricular extrasystoles, and first-degree atrioventricular block, were detected in the CTSL-deficient mice.
Figures



References
-
- Kamisago M, Sharma S D, DePalma S R, Solomon S, Sharma P, McDonough B, Smoot L, Mullen M P, Woolf P K, Wigle E D, et al. N Engl J Med. 2000;343:1688–1696. - PubMed
-
- Olson T M, Michels V V, Thibodeau S N, Tai Y S, Keating M T. Science. 1998;280:750–752. - PubMed
-
- Li D, Tapscoft T, Gonzalez O, Burch P E, Quinones M A, Zoghbi W A, Hill R, Bachinski L L, Mann D L, Roberts R. Circulation. 1999;100:461–464. - PubMed
-
- Towbin J A, Hejtmancik J F, Brink P, Gelb B, Zhu X M, Chamberlain J S, McCabe E R, Swift M. Circulation. 1993;87:1854–1865. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

