Extracellular polysaccharides of Rhodococcus rhodochrous S-2 stimulate the degradation of aromatic components in crude oil by indigenous marine bacteria
- PMID: 11976106
- PMCID: PMC127525
- DOI: 10.1128/AEM.68.5.2337-2343.2002
Extracellular polysaccharides of Rhodococcus rhodochrous S-2 stimulate the degradation of aromatic components in crude oil by indigenous marine bacteria
Abstract
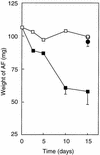
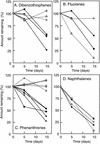
Rhodococcus rhodochrous S-2 produces extracellular polysaccharides (S-2 EPS) containing D-glucose, D-galactose, D-mannose, D-glucuronic acid, and lipids, which is important to the tolerance of this strain to an aromatic fraction of (AF) Arabian light crude oil (N. Iwabuchi, N. Sunairi, H. Anzai, M. Nakajima, and S. Harayama, Appl. Environ. Microbiol. 66:5073-5077, 2000). In the present study, we examined the effects of S-2 EPS on the growth of indigenous marine bacteria on AF. Indigenous bacteria did not grow significantly in seawater containing AF even when nitrogen, phosphorus, and iron nutrients were supplemented. The addition of S-2 EPS to seawater containing nutrients and AF resulted in the emulsification of AF, promotion of the growth of indigenous bacteria, and enhancement of the degradation of AF by the bacteria. PCR-denaturing gradient gel electrophoresis analyses show that addition of S-2 EPS to the seawater containing nutrients and AF changed the composition of the bacterial populations in the seawater and that bacteria closely related to the genus Cycloclasticus became the major population. These results suggest that Cycloclasticus was responsible for the degradation of hydrocarbons in AF. The effects of 15 synthetic surfactants on the degradation of AF by indigenous marine bacteria were also examined, but enhancement of the degradation of AF was not significant. S-2 EPS was hence the most effective of the surfactants tested in promoting the biodegradation of AF and may thus be an attractive agent to use in the bioremediation of oil-contaminated marine environments.
Figures


References
-
- Bartha, R. 1993. Microbial ecology: fundamentals and applications, 3rd ed. Addison Wesley Longman Publishers, Amsterdam, The Netherlands.
-
- Bell, K. S., J. C. Philip, D. W. J. Aw, and N. Christofi. 1998. The genus Rhodococcus. J. Appl. Microbiol. 85:195-210. - PubMed
-
- Dutta, T. K., and S. Harayama. 2000. Fate of crude oil by the combination of photooxidation and biodegradation. Environ. Sci. Technol. 34:1500-1505.
-
- Dyksterhouse, S. E., J. P. Gray, R. P. Herwig, J. C. Lara, and J. T. Staley. 1995. Cycloclasticus pugetii gen. nov., sp. nov., an aromatic hydrocarbon-degrading bacterium from marine sediments. Int. J. Syst. Bacteriol. 45:116-123. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

