Alterations in Vibrio fischeri motility correlate with a delay in symbiosis initiation and are associated with additional symbiotic colonization defects
- PMID: 11976129
- PMCID: PMC127559
- DOI: 10.1128/AEM.68.5.2519-2528.2002
Alterations in Vibrio fischeri motility correlate with a delay in symbiosis initiation and are associated with additional symbiotic colonization defects
Abstract
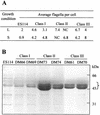
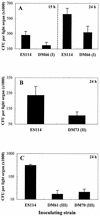
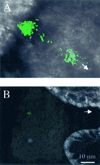
Motility is required for Vibrio fischeri cells to interact with and specifically colonize the light-emitting organ of their host, the squid Euprymna scolopes. To investigate the influence of motility on the expression of the symbiotic phenotype, we isolated mutants of the squid symbiont V. fischeri ES114 that had altered migration abilities. Spontaneous hyperswimmer (HS) mutants, which migrated more rapidly in soft agar and were hyperflagellated relative to the wild type, were isolated and grouped into three phenotypic classes. All of the HS strains tested, regardless of class, were delayed in symbiosis initiation. This result suggested that the hypermotile phenotype alone contributes to an inability to colonize squid normally. Class III HS strains showed the greatest colonization defect: they colonized squid to a level that was only 0.1 to 10% that achieved by ES114. In addition, class III strains were defective in two capabilities, hemagglutination and luminescence, that have been previously described as colonization factors in V. fischeri. Class II and III mutants also share a mucoid colony morphology; however, class II mutants can colonize E. scolopes to a level that was 40% of that achieved by ES114. Thus, the mucoid phenotype alone does not contribute to the greater defect exhibited by class III strains. When squid were exposed to ES114 and any one of the HS mutant strains as a coinoculation, the parent strain dominated the resulting symbiotic light-organ population. To further investigate the colonization defects of the HS strains, we used confocal laser-scanning microscopy to visualize V. fischeri cells in their initial interaction with E. scolopes tissue. Compared to ES114, HS strains from all three classes were delayed in two behaviors involved in colonization: (i) aggregation on host-derived mucus structures and (ii) migration to the crypts. These results suggest that, while motility is required to initiate colonization, the presence of multiple flagella may actually interfere with normal aggregation and attachment behavior. Furthermore, the pleiotropic nature of class III HS strains provides evidence that motility is coregulated with other symbiotic determinants in V. fischeri.
Figures



References
-
- Akerley, B. J., and J. F. Miller. 1996. Understanding signal transduction during bacterial infection. Trends Microbiol. 4:141-146. - PubMed
-
- Ali, A., J. A. Johnson, A. A. Franco, D. J. Metzger, T. D. Connell, J. G. Morris, Jr., and S. Sozhamannan. 2000. Mutations in the extracellular protein secretion pathway genes (eps) interfere with rugose polysaccharide production in and motility of Vibrio cholerae. Infect. Immun. 68:1967-1974. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

