Pharmacology of the nitric oxide receptor, soluble guanylyl cyclase, in cerebellar cells
- PMID: 11976273
- PMCID: PMC1762114
- DOI: 10.1038/sj.bjp.0704687
Pharmacology of the nitric oxide receptor, soluble guanylyl cyclase, in cerebellar cells
Abstract
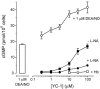
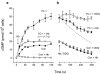
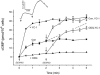
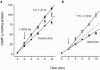
The nitric oxide (NO) receptor, soluble guanylyl cyclase (sGC), is commonly manipulated pharmacologically in two ways. Inhibition of activity is achieved using 1-H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-l-one (ODQ) which oxidizes the haem prosthetic group to which NO binds, while the compound 3-(5-hydroxymethyl-2-furyl)-1-benzylindazole (YC-1) is considered an 'allosteric' activator. Knowledge of how these agents function and interact in a normal cellular environment is limited. These issues were addressed using rat cerebellar cells. Inhibition by ODQ was not simply competitive with NO. The rate of onset was ODQ concentration-dependent and developed in two kinetic phases. Recovery from inhibition occurred with a half-time of approximately 5 min. YC-1 slowed the rate at which sGC deactivated on removal of NO by 45 fold, consistent with YC-1 increasing the potency of NO for sGC. YC-1 also enhanced the maximal response to NO by 2 fold. Furthermore, when added to cells in which sGC was 90% desensitized, YC-1 abruptly enhanced sGC activity to a degree that indicated partial reversal of desensitization. After pre-exposure to YC-1, sGC became resistant to inhibition by ODQ. In addition, YC-1 rapidly reversed inhibition by ODQ in cells and for purified sGC, suggesting that YC-1 either increases the NO affinity of the oxidized sGC haem or reverses haem oxidation. It is concluded that the actions of ODQ and YC-1 on sGC are broadly similar in cells and purified preparations. Additionally, YC-1 transiently reverses sGC desensitization in cells. It is hypothesized that YC-1 has multiple actions on sGC, and thereby both modifies the NO binding site and enhances agonist efficacy.
Figures


Similar articles
-
Effects of the soluble guanylyl cyclase activator, YC-1, on vascular tone, cyclic GMP levels and phosphodiesterase activity.Br J Pharmacol. 1999 May;127(1):195-203. doi: 10.1038/sj.bjp.0702495. Br J Pharmacol. 1999. PMID: 10369473 Free PMC article.
-
Investigation of the vasorelaxant effects of 3-(5'-hydroxymethyl-2'-furyl)-1-benzyl indazole (YC-1) and diethylamine/nitric oxide (DEA/NO) on the human radial artery used as coronary bypass graft.Can J Physiol Pharmacol. 2007 May;85(5):521-6. doi: 10.1139/y07-033. Can J Physiol Pharmacol. 2007. PMID: 17632587
-
YC-1 activation of human soluble guanylyl cyclase has both heme-dependent and heme-independent components.Proc Natl Acad Sci U S A. 2001 Nov 6;98(23):12938-42. doi: 10.1073/pnas.231486198. Epub 2001 Oct 30. Proc Natl Acad Sci U S A. 2001. PMID: 11687640 Free PMC article.
-
NO-independent, haem-dependent soluble guanylate cyclase stimulators.Handb Exp Pharmacol. 2009;(191):277-308. doi: 10.1007/978-3-540-68964-5_13. Handb Exp Pharmacol. 2009. PMID: 19089334 Review.
-
The receptor-like properties of nitric oxide-activated soluble guanylyl cyclase in intact cells.Mol Cell Biochem. 2002 Jan;230(1-2):165-76. Mol Cell Biochem. 2002. PMID: 11952092 Review.
Cited by
-
Voluntary wheel running activates Akt/AMPK/eNOS signaling cascades without improving profound endothelial dysfunction in mice deficient in α-galactosidase A.PLoS One. 2019 May 23;14(5):e0217214. doi: 10.1371/journal.pone.0217214. eCollection 2019. PLoS One. 2019. PMID: 31120949 Free PMC article.
-
Inhibition of nitric oxide-activated guanylyl cyclase by calmodulin antagonists.Br J Pharmacol. 2009 Nov;158(6):1454-64. doi: 10.1111/j.1476-5381.2009.00416.x. Epub 2009 Oct 20. Br J Pharmacol. 2009. PMID: 19845679 Free PMC article.
-
Inactivation of soluble guanylyl cyclase in living cells proceeds without loss of haem and involves heterodimer dissociation as a common step.Br J Pharmacol. 2022 Jun;179(11):2505-2518. doi: 10.1111/bph.15527. Epub 2021 Jun 16. Br J Pharmacol. 2022. PMID: 33975383 Free PMC article.
-
Probing the presence of the ligand-binding haem in cellular nitric oxide receptors.Br J Pharmacol. 2008 Apr;153(7):1495-504. doi: 10.1038/sj.bjp.0707687. Epub 2008 Jan 21. Br J Pharmacol. 2008. PMID: 18204474 Free PMC article.
-
Nitric oxide stimulation of cGMP accumulation in myometrial cells from pregnant women is antagonized by oxytocin.Proc West Pharmacol Soc. 2008;51:78-82. Proc West Pharmacol Soc. 2008. PMID: 19544684 Free PMC article.
References
-
- BELLAMY T.C., GARTHWAITE J. ‘cAMP-specific' phosphodiesterase contributes to cGMP degradation in cerebellar cells exposed to nitric oxide. Mol. Pharmacol. 2001a;59:54–61. - PubMed
-
- BELLAMY T.C., GARTHWAITE J. Sub-second kinetics of the nitric oxide receptor, soluble guanylyl cyclase, in intact cerebellar cells. J. Biol. Chem. 2001b;276:4287–4292. - PubMed
-
- COLQUHOUN D., HAWKES A.G.The principles of the stochastic interpretation of ion-channel mechanisms Single-Channel Recording 1995New York: Plenum Press; 397–482.ed. B. Sakmann & E. Neher. pp
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

