Thioridazine interacts with the membrane of mitochondria acquiring antioxidant activity toward apoptosis--potentially implicated mechanisms
- PMID: 11976278
- PMCID: PMC1762107
- DOI: 10.1038/sj.bjp.0704672
Thioridazine interacts with the membrane of mitochondria acquiring antioxidant activity toward apoptosis--potentially implicated mechanisms
Abstract
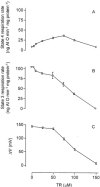
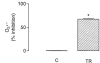
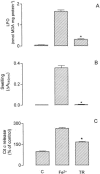
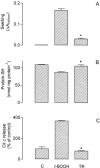
We evaluated the effects of the phenothiazine derivative thioridazine on mechanisms of mitochondria potentially implicated in apoptosis, such as those involving reactive oxygen species (ROS) and cytochrome c release, as well as the involvement of drug interaction with mitochondrial membrane in these effects. Within the 0 - 100 microM range thioridazine did not reduce the free radical 1,1-diphenyl-2-picryl-hydrazyl (DPPH) nor did it chelate iron. However, at 10 microM thioridazine showed important antioxidant activity on mitochondria, characterized by inhibition of accumulation of mitochondria-generated O2*-, assayed as lucigenin-derived chemiluminescence, inhibition of Fe2+/citrate-mediated lipid peroxidation of the mitochondrial membrane (LPO), assayed as malondialdehyde generation, and inhibition of Ca2+/t-butyl hydroperoxide (t-BOOH)-induced mitochondrial permeability transition (MPT)/protein-thiol oxidation, assayed as mitochondrial swelling. Thioridazine respectively increased and decreased the fluorescence responses of mitochondria labelled with 1-aniline-8-naphthalene sulfonate (ANS) and 1-(4-trimethylammonium phenyl)-6 phenyl 1,3,5-hexatriene (TMA-DPH). The inhibition of LPO and MPT onset correlated well with the inhibition of cytochrome c release from mitochondria. We conclude that thioridazine interacts with the inner membrane of mitochondria, more likely close to its surface, acquiring antioxidant activity toward processes with potential implications in apoptosis such as O2*- accumulation, as well as LPO, MPT and associated release of cytochrome c.
Figures
Similar articles
-
Mangiferin, a natural occurring glucosyl xanthone, increases susceptibility of rat liver mitochondria to calcium-induced permeability transition.Arch Biochem Biophys. 2005 Jul 15;439(2):184-93. doi: 10.1016/j.abb.2005.05.015. Arch Biochem Biophys. 2005. PMID: 15979560
-
Effects of isocoumarins isolated from Paepalanthus bromelioides on mitochondria: uncoupling, and induction/inhibition of mitochondrial permeability transition.Chem Biol Interact. 2006 Jun 10;161(2):155-64. doi: 10.1016/j.cbi.2006.04.006. Epub 2006 Apr 28. Chem Biol Interact. 2006. PMID: 16716282
-
Oxidative stress underlies the mechanism for Ca(2+)-induced permeability transition of mitochondria.Free Radic Res. 2004 Jan;38(1):27-35. doi: 10.1080/10715760310001626266. Free Radic Res. 2004. PMID: 15061651
-
The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy.Biochim Biophys Acta. 1998 Aug 10;1366(1-2):177-96. doi: 10.1016/s0005-2728(98)00112-1. Biochim Biophys Acta. 1998. PMID: 9714796 Review.
-
Involvement of mitochondrial phospholipid hydroperoxide glutathione peroxidase as an antiapoptotic factor.Biol Signals Recept. 2001 Jan-Apr;10(1-2):81-92. doi: 10.1159/000046877. Biol Signals Recept. 2001. PMID: 11223642 Review.
Cited by
-
Specific effects of reactive thiol drugs on mitochondrial bioenergetics.J Bioenerg Biomembr. 2011 Feb;43(1):11-8. doi: 10.1007/s10863-011-9328-9. J Bioenerg Biomembr. 2011. PMID: 21279427 Review.
-
Evaluation of functional stability of quercetin as a raw material and in different topical formulations by its antilipoperoxidative activity.AAPS PharmSciTech. 2006 Mar;7(1):E64-E71. doi: 10.1208/pt070110. Epub 2017 Mar 8. AAPS PharmSciTech. 2006. PMID: 28290025 Free PMC article.
-
4-methylcoumarin derivatives inhibit human neutrophil oxidative metabolism and elastase activity.J Med Food. 2013 Aug;16(8):692-700. doi: 10.1089/jmf.2012.0184. Epub 2013 Aug 1. J Med Food. 2013. PMID: 23905650 Free PMC article.
-
NOX4-mediated ROS production induces apoptotic cell death via down-regulation of c-FLIP and Mcl-1 expression in combined treatment with thioridazine and curcumin.Redox Biol. 2017 Oct;13:608-622. doi: 10.1016/j.redox.2017.07.017. Epub 2017 Aug 9. Redox Biol. 2017. PMID: 28806703 Free PMC article.
-
Mitochondria DNA change and oxidative damage in clinically stable patients with major depressive disorder.PLoS One. 2015 May 6;10(5):e0125855. doi: 10.1371/journal.pone.0125855. eCollection 2015. PLoS One. 2015. PMID: 25946463 Free PMC article.
References
-
- ÅKERMAN K.E.O., WIKSTRÖM M.K.F. Safranine as a probe of the mitochondrial membrane potential. FEBS Lett. 1976;68:191–197. - PubMed
-
- BALDESSARINI R.J.Drugs and the treatment of psychiatric disorders: psychosis and anxiety Goodman & Gilman's The Pharmacological Basis of Therapeutics 1995New York: McGraw-Hill; 399–430.9th edn ed. Hardman, J.G., Gilman, A.G. & Limbird, L.E., pp
-
- BERNARDI P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol. Rev. 1999;79:1127–1155. - PubMed
-
- BERNARDI P., PETRONILLI V., DI LISA F., FORTE M. A mitochondrial perspective on cell death. Trends Biochem. Sci. 2001;26:112–117. - PubMed
-
- BLOIS M.S. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

