The E1beta and E2 subunits of the Bacillus subtilis pyruvate dehydrogenase complex are involved in regulation of sporulation
- PMID: 11976308
- PMCID: PMC135025
- DOI: 10.1128/JB.184.10.2780-2788.2002
The E1beta and E2 subunits of the Bacillus subtilis pyruvate dehydrogenase complex are involved in regulation of sporulation
Abstract
The pdhABCD operon of Bacillus subtilis encodes the pyruvate decarboxylase (E1alpha and E1beta), dihydrolipoamide acetyltransferase (E2), and dihydrolipoamide dehydrogenase (E3) subunits of the pyruvate dehydrogenase multienzyme complex (PDH). There are two promoters: one for the entire operon and an internal one in front of the pdhC gene. The latter may serve to ensure adequate quantities of the E2 and E3 subunits, which are needed in greater amounts than E1alpha and E1beta. Disruptions of the pdhB, pdhC, and pdhD genes were isolated, but attempts to construct a pdhA mutant were unsuccessful, suggesting that E1alpha is essential. The three mutants lacked PDH activity, were unable to grow on glucose and grew poorly in an enriched medium. The pdhB and pdhC mutants sporulated to only 5% of the wild-type level, whereas the pdhD mutant strain sporulated to 55% of the wild-type level. This difference indicated that the sporulation defect of the pdhB and pdhC mutant strains was due to a function(s) of these subunits independent of enzymatic activity. Growth, but not low sporulation, was enhanced by the addition of acetate, glutamate, succinate, and divalent cations. Results from the expression of various spo-lacZ fusions revealed that the pdhB mutant was defective in the late stages of engulfment or membrane fusion (stage II), whereas the pdhC mutant was blocked after the completion of engulfment (stage III). This analysis was confirmed by fluorescent membrane staining. The E1beta and E2 subunits which are present in the soluble fraction of sporulating cells appear to function independently of enzymatic activity as checkpoints for stage II-III of sporulation.
Figures



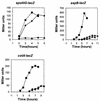
 ), 501-77 (ΔpdhC; ▴), and 501-78 (ΔpdhD; ×). For each strain, the starting cell concentration was adjusted to 2 Klett units from an overnight culture.
), 501-77 (ΔpdhC; ▴), and 501-78 (ΔpdhD; ×). For each strain, the starting cell concentration was adjusted to 2 Klett units from an overnight culture.


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

