Critical role of the hippocampus in memory for sequences of events
- PMID: 11976705
- PMCID: PMC4053170
- DOI: 10.1038/nn834
Critical role of the hippocampus in memory for sequences of events
Abstract
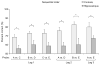
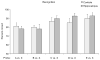
Recent models of hippocampal function emphasize the potential role of this brain structure in encoding and retrieving sequences of events that compose episodic memories. Here we show that hippocampal lesions produce a severe and selective impairment in the capacity of rats to remember the sequential ordering of a series of odors, despite an intact capacity to recognize odors that recently occurred. These findings support the hypothesis that hippocampal networks mediate associations between sequential events that constitute elements of an episodic memory.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures



References
-
- Vargha-Khadem F, et al. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–380. - PubMed
-
- Tulving E, Markowitsch HJ. Episodic and declarative memory: role of the hippocampus. Hippocampus. 1998;8:198–204. - PubMed
-
- Levy WB. A sequence predicting CA3 is a flexible associator that learns and uses context to solve hippocampal-like tasks. Hippocampus. 1996;6:579–590. - PubMed
-
- Sohal VS, Hasselmo ME. Changes in GABAB modulation during a theta cycle may be analogous to the fall of temperature during annealing. Neural Comput. 1998;10:889–902. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

