Functional stoichiometry of glutamate receptor desensitization
- PMID: 11978816
- PMCID: PMC6758362
- DOI: 10.1523/JNEUROSCI.22-09-03392.2002
Functional stoichiometry of glutamate receptor desensitization
Abstract
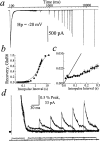
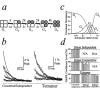
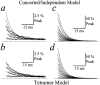
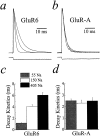
Potassium (K+) channels and ionotropic glutamate receptors (iGluRs) fulfill divergent roles in vertebrate nervous systems. Despite this, however, recent work suggests that these ion channels are structurally homologous, sharing an ancestral protein, architectural design, and tetrameric subunit stoichiometry. Their gating mechanisms also are speculated to have overlapping features. Here we show that the mechanism of iGluR desensitization is unique. Unlike K+ channels, AMPA- and kainate-type iGluR subunits desensitize in several ordered conformational steps. AMPA receptors operate as dimers, whereas the functional stoichiometry of kainate receptor desensitization is dependent on external ions. Contrary to conventional understanding, kinetic models suggest that partially desensitized AMPA and kainate receptors conduct ions and are likely participants in synaptic signaling. Although sharing many structural correlates with K+ channels, iGluRs have evolved unique subunit-subunit interactions, tailoring their gating behavior to fulfill distinct roles in neuronal signaling.
Figures


References
-
- Armstrong N, Gouaux E. Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron. 2000;28:165–181. - PubMed
-
- Armstrong N, Sun Y, Chen G-Q, Gouaux E. Structure of a glutamate receptor ligand-binding core in complex with kainate. Nature. 1998;395:913–917. - PubMed
-
- Ayalon G, Stern-Bach Y. Functional assembly of AMPA and kainate receptors is mediated by several discrete protein–protein interactions. Neuron. 2001;31:103–113. - PubMed
-
- Baukrowitz T, Yellen G. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron. 1995;15:951–960. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
