Lentiviral-mediated delivery of mutant huntingtin in the striatum of rats induces a selective neuropathology modulated by polyglutamine repeat size, huntingtin expression levels, and protein length
- PMID: 11978824
- PMCID: PMC6758353
- DOI: 10.1523/JNEUROSCI.22-09-03473.2002
Lentiviral-mediated delivery of mutant huntingtin in the striatum of rats induces a selective neuropathology modulated by polyglutamine repeat size, huntingtin expression levels, and protein length
Abstract
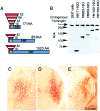
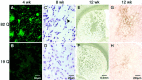
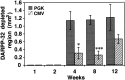
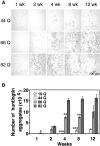
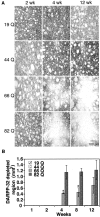
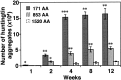
A new strategy based on lentiviral-mediated delivery of mutant huntingtin (htt) was used to create a genetic model of Huntington's disease (HD) in rats and to assess the relative contribution of polyglutamine (CAG) repeat size, htt expression levels, and protein length on the onset and specificity of the pathology. Lentiviral vectors coding for the first 171, 853, and 1520 amino acids of wild-type (19 CAG) or mutant htt (44, 66, and 82 CAG) driven by either the phosphoglycerate kinase 1 (PGK) or the cytomegalovirus (CMV) promoters were injected in rat striatum. A progressive pathology characterized by sequential appearance of ubiquitinated htt aggregates, loss of dopamine- and cAMP-regulated phosphoprotein of 32 kDa staining, and cell death was observed over 6 months with mutant htt. Earlier onset and more severe pathology occurred with shorter fragments, longer CAG repeats, and higher expression levels. Interestingly, the aggregates were predominantly located in the nucleus of PGK-htt171-injected rats, whereas they were present in both the nucleus and processes of CMV-htt171-injected animals expressing lower transgene levels. Finally, a selective sparing of interneurons was observed in animals injected with vectors expressing mutant htt. These data demonstrate that lentiviral-mediated expression of mutant htt provides a robust in vivo genetic model for selective neural degeneration that will facilitate future studies on the pathogenesis of cell death and experimental therapeutics for HD.
Figures





References
-
- Andrew SE, Goldberg YP, Kremer B, Telenius H, Theilmann J, Adam S, Starr E, Squitieri F, Lin B, Kalchman MA, Graham RK, Hayden MR. The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington's disease. Nat Genet. 1993;4:398–403. - PubMed
-
- Andrews TC, Weeks RA, Turjanski N, Gunn RN, Watkins LH, Sahakian B, Hodges JR, Rosser AE, Wood NW, Brooks DJ. Huntington's disease progression. PET and clinical observations. Brain. 1999;122:2353–2363. - PubMed
-
- Antonini A, Leenders KL, Spiegel R, Meier D, Vontobel P, Weigell-Weber M, Sanchez-Pernaute R, de Yebenez JG, Boesiger P, Weindl A, Maguire RP. Striatal glucose metabolism and dopamine D2 receptor binding in asymptomatic gene carriers and patients with Huntington's disease. Brain. 1996;119:2085–2095. - PubMed
-
- Aylward EH, Brandt J, Codori AM, Mangus RS, Barta PE, Harris GJ. Reduced basal ganglia volume associated with the gene for Huntington's disease in asymptomatic at-risk persons. Neurology. 1994;44:823–828. - PubMed
-
- Aylward EH, Codori AM, Rosenblatt A, Sherr M, Brandt J, Stine OC, Barta PE, Pearlson GD, Ross CA. Rate of caudate atrophy in presymptomatic and symptomatic stages of Huntington's disease. Mov Disord. 2000;15:552–560. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
