Muscle spindle-derived neurotrophin 3 regulates synaptic connectivity between muscle sensory and motor neurons
- PMID: 11978828
- PMCID: PMC6758400
- DOI: 10.1523/JNEUROSCI.22-09-03512.2002
Muscle spindle-derived neurotrophin 3 regulates synaptic connectivity between muscle sensory and motor neurons
Abstract
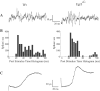
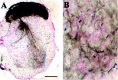
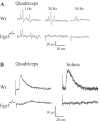
Ia afferents induce the formation of muscle spindles prenatally and maintain them postnatally. To address whether spindles, in turn, regulate the function of Ia afferents, we examined Egr3-null mutant mice (Egr3-/-), in which muscle spindles degenerate progressively after birth. Egr3-/- mice develop gait ataxia, scoliosis, resting tremors, and ptosis, suggesting a defect in proprioception. Despite the normal morphological appearance of peripheral and central sensory projections, we observed a profound functional deficit in the strength of sensory-motor connections in Egr3-/- mice. Muscle spindles in Egr3-/- mice do not express NT3. Intramuscular injections of NT3 to Egr3-/- mice during the postnatal period restored sensory-motor connections. Thus, NT3 derived from muscle spindles regulates the synaptic connectivity between muscle sensory and motor neurons.
Figures





References
-
- Arber S, Ladle DR, Lin JH, Frank E, Jessell TM. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell. 2000;101:485–498. - PubMed
-
- Arvanov VL, Seebach BS, Mendell LM. NT-3 evokes an LTP-like facilitation of AMPA/kainate receptor-mediated synaptic transmission in the neonatal rat spinal cord. J Neurophysiol. 2000;84:752–758. - PubMed
-
- Blasi J, Chapman ER, Link E, Binz T, Yamasaki S, De Camilli P, Sudhof TC, Niemann H, Jahn R. Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature. 1993;365:160–163. - PubMed
-
- Carr VM, Simpson SB., Jr Proliferative and degenerative events in the early development of chick dorsal root ganglia. I. Normal development. J Comp Neurol. 1978;182:727–739. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
