Deleted in colorectal carcinoma and differentially expressed integrins mediate the directional migration of neural precursors in the rostral migratory stream
- PMID: 11978833
- PMCID: PMC6758349
- DOI: 10.1523/JNEUROSCI.22-09-03568.2002
Deleted in colorectal carcinoma and differentially expressed integrins mediate the directional migration of neural precursors in the rostral migratory stream
Abstract
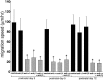
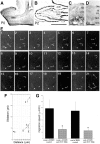
Precursors of the olfactory interneurons migrate from the subventricular zone via the rostral migratory stream (RMS). To investigate the molecular mechanisms by which RMS cells migrate, we used a slice preparation, which allows the migrating cells to be imaged at very high temporal and spatial resolution in the presence of added inhibitors. Using immunohistochemistry, we first determined that the alpha1-, beta8-, and beta1-integrin subunits and the alpha5- and gamma1-laminin subunits are expressed during embryonic day 16 to the early postnatal stage. During early postnatal days, alpha(v)- and beta6-integrins appeared, and their expression persisted throughout adulthood. The migrating cells also expressed the netrin receptors neogenin and Deleted in Colorectal Carcinoma (DCC). Netrin-1 is expressed in olfactory mitral cells. Anti-integrin antibodies inhibited the production of protrusions as well as cellular translocation. In contrast, anti-DCC antibodies primarily altered the direction of the protrusions; consequently, the migration was no longer unidirectional, and the speed was reduced. Thus, the interaction of DCC, possibly through an interaction with netrin-1, contributes to the direction of migration by regulating the formation of directed protrusions. In contrast, the integrins function in production of protrusions and cellular translocation, with different integrins participating at different developmental stages.
Figures





Similar articles
-
Doublecortin expression in the adult rat telencephalon.Eur J Neurosci. 2001 Aug;14(4):629-44. doi: 10.1046/j.0953-816x.2001.01683.x. Eur J Neurosci. 2001. PMID: 11556888
-
Expression of drebrin E in migrating neuroblasts in adult rat brain: coincidence between drebrin E disappearance from cell body and cessation of migration.Neuroscience. 2008 Mar 27;152(3):670-82. doi: 10.1016/j.neuroscience.2007.10.068. Epub 2008 Jan 19. Neuroscience. 2008. PMID: 18304746
-
Protein expression differs between neural progenitor cells from the adult rat brain subventricular zone and olfactory bulb.BMC Neurosci. 2008 Jan 16;9:7. doi: 10.1186/1471-2202-9-7. BMC Neurosci. 2008. PMID: 18197988 Free PMC article.
-
Dynamic changes in the transcriptional profile of subventricular zone-derived postnatally born neuroblasts.Mech Dev. 2013 Jun-Aug;130(6-8):424-32. doi: 10.1016/j.mod.2012.11.003. Epub 2012 Dec 5. Mech Dev. 2013. PMID: 23220001 Review.
-
The rostral migratory stream and olfactory system: smell, disease and slippery cells.Prog Brain Res. 2009;175:33-42. doi: 10.1016/S0079-6123(09)17503-9. Prog Brain Res. 2009. PMID: 19660647 Review.
Cited by
-
The Extracellular Matrix Glycoprotein Tenascin C and Adult Neurogenesis.Front Cell Dev Biol. 2021 Apr 29;9:674199. doi: 10.3389/fcell.2021.674199. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33996833 Free PMC article. Review.
-
Involvement of headless myosin X in the motility of immortalized gonadotropin-releasing hormone neuronal cells.Cell Biol Int. 2009 May;33(5):578-85. doi: 10.1016/j.cellbi.2009.02.006. Epub 2009 Feb 28. Cell Biol Int. 2009. PMID: 19254772 Free PMC article.
-
A symphony of signals conducts early and late stages of adult neurogenesis.Neuropharmacology. 2010 May;58(6):865-76. doi: 10.1016/j.neuropharm.2010.01.010. Epub 2010 Jan 25. Neuropharmacology. 2010. PMID: 20097213 Free PMC article. Review.
-
The role of Rho GTPase proteins in CNS neuronal migration.Dev Neurobiol. 2011 Jun;71(6):528-53. doi: 10.1002/dneu.20850. Dev Neurobiol. 2011. PMID: 21557504 Free PMC article. Review.
-
Beyond the Hippocampus and the SVZ: Adult Neurogenesis Throughout the Brain.Front Cell Neurosci. 2020 Sep 29;14:576444. doi: 10.3389/fncel.2020.576444. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33132848 Free PMC article. Review.
References
-
- Allen E. The cessation of mitosis in the central nervous system of the albino rat. J Comp Neurol. 1912;22:547–568.
-
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–457. - PubMed
-
- Boulder Committee: Angevine JBJ, Bodian D, Coulombre AJ, Edds MVJ, Hamburger V, Jacobson M, Lyser KM, Prestige MC, Sidman RL, Varon S, Weiss PA. Embryonic vertebrate central nervous system: revised terminology. Anat Rec. 1970;166:257–261. - PubMed
-
- Bryans WA. Mitotic activity in the brain of the adult rat. Anat Rec. 1959;133:65–73.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
