Increased neurogenesis in adult mCD24-deficient mice
- PMID: 11978835
- PMCID: PMC6758384
- DOI: 10.1523/JNEUROSCI.22-09-03594.2002
Increased neurogenesis in adult mCD24-deficient mice
Abstract
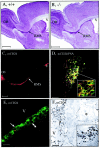
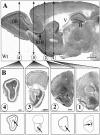
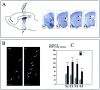
mCD24, a glycosylphosphatidylinositol-anchored highly glycosylated molecule, is expressed on differentiating neurons during development. In the adult CNS, its expression is restricted to immature neurons located in two regions showing ongoing neurogenesis: the subventricular zone (SVZ) of the lateral ventricle pathway and the dentate gyrus (DG) of the hippocampal formation. Here, combining bromodeoxyuridine (BrdU) and proliferating cell nuclear antigen labelings we confirmed that mCD24 is expressed on proliferating cells. To determine whether the inactivation of the molecule may affect adult neurogenesis, we analyzed the phenotype of mCD24-deficient mice (mCD24-/-). We labeled cells in S-phase with a pulse, a long, or a cumulative administration of BrdU and analyzed cells in different zones according to their dividing rate (rapid and slow) both in the control and mCD24-/-. We found a significant increase in the number of rapid (in the SVZ and the DG) and slow (in the SVZ) proliferating cells. Cumulative assays revealed a global reduction of the total cell cycle duration of rapidly proliferating precursors of SVZ. We investigated the fate of supernumerary cells and observed an increased number of apoptotic cells (terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling) in the mutant SVZ. Furthermore, we found no difference in the size of the olfactory bulb between wild-type (WT) and mutant mice. In support, mCD24 deletion did not appear to affect migration in the migratory stream. A comparison of the organization of migrating precursors between WT and mCD24 -/-, both in vivo at the optic and electron microscopic levels and in SVZ cultured explants, did not show any changes in the arrangement of neuroblasts in chain-like structures. Altogether, our data suggest that mCD24 regulates negatively cell proliferation in zones of secondary neurogenesis.
Figures






References
-
- Aigner S, Sthoeger ZM, Fogel M, Weber E, Zarn J, Ruppert M, Zeller Y, Vestweber D, Stahel R, Sammar M, Altevogt P. CD24, a murin-type glycoprotein, is a ligand for P-selectin on human tumor cells. Blood. 1997;89:3385–3395. - PubMed
-
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. 3. Dating the time of production and onset of differentiation of cerebellar microneurons in rats. J Comp Neurol. 1969;136:269–293. - PubMed
-
- Altman J, Das GD. Post-natal origin of microneurons in the rat brain. Nature. 1965;207:953–956. - PubMed
-
- Biebl M, Cooper CM, Winkler J, Kuhn HG. Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci Lett. 2000;291:17–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
