Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein
- PMID: 11978843
- PMCID: PMC6758358
- DOI: 10.1523/JNEUROSCI.22-09-03673.2002
Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein
Abstract
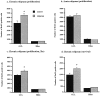
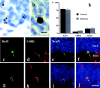
The cAMP cascade, including the cAMP response element-binding protein (CREB), is known to play an important role in neuronal survival and plasticity. Here the influence of this cascade on neurogenesis in adult hippocampus was determined. Activation of the cAMP cascade by administration of rolipram, an inhibitor of cAMP breakdown, increased the proliferation of newborn cells in adult mouse hippocampus. In addition, rolipram induction of cell proliferation resulted in mature granule cells that express neuronal-specific markers. Increased cell proliferation is accompanied by activation of CREB phosphorylation in dentate gyrus granule cells, suggesting a role for this transcription factor. This possibility is supported by studies demonstrating that cell proliferation is decreased in conditional transgenic mice that express a dominant negative mutant of CREB in hippocampus. The results suggest that the cAMP-CREB cascade could contribute to the actions of neurotransmitters and neurotrophic factors on adult neurogenesis.
Figures




Similar articles
-
Activation of cAMP signaling facilitates the morphological maturation of newborn neurons in adult hippocampus.J Neurosci. 2004 Jan 14;24(2):319-28. doi: 10.1523/JNEUROSCI.1065.03.2004. J Neurosci. 2004. PMID: 14724230 Free PMC article.
-
The phosphodiesterase inhibitor rolipram promotes survival of newborn hippocampal neurons after ischemia.Stroke. 2007 May;38(5):1597-605. doi: 10.1161/STROKEAHA.106.476754. Epub 2007 Mar 22. Stroke. 2007. PMID: 17379823
-
Localization of phosphorylated cAMP response element-binding protein in immature neurons of adult hippocampus.J Neurosci. 2002 Nov 15;22(22):9868-76. doi: 10.1523/JNEUROSCI.22-22-09868.2002. J Neurosci. 2002. PMID: 12427843 Free PMC article.
-
Is CREB a key to neuronal survival?Trends Neurosci. 2000 Feb;23(2):48-53. doi: 10.1016/s0166-2236(99)01500-3. Trends Neurosci. 2000. PMID: 10652539 Review.
-
A new perspective on the role of the CREB family of transcription factors in memory consolidation via adult hippocampal neurogenesis.Front Mol Neurosci. 2015 Aug 26;8:46. doi: 10.3389/fnmol.2015.00046. eCollection 2015. Front Mol Neurosci. 2015. PMID: 26379491 Free PMC article. Review.
Cited by
-
The Role of Neurotrophic Factors in Pathophysiology of Major Depressive Disorder.Adv Exp Med Biol. 2021;1305:257-272. doi: 10.1007/978-981-33-6044-0_14. Adv Exp Med Biol. 2021. PMID: 33834404
-
Vascular endothelial growth factor regulates adult hippocampal cell proliferation through MEK/ERK- and PI3K/Akt-dependent signaling.Neuropharmacology. 2012 Sep;63(4):642-52. doi: 10.1016/j.neuropharm.2012.04.033. Epub 2012 May 10. Neuropharmacology. 2012. PMID: 22580375 Free PMC article.
-
Signaling mechanisms regulating adult neural stem cells and neurogenesis.Biochim Biophys Acta. 2013 Feb;1830(2):2435-48. doi: 10.1016/j.bbagen.2012.09.002. Epub 2012 Sep 12. Biochim Biophys Acta. 2013. PMID: 22982587 Free PMC article. Review.
-
Serotonin 1A receptor agonist increases species- and region-selective adult CNS proliferation, but not through CNTF.Neuropharmacology. 2012 Dec;63(7):1238-47. doi: 10.1016/j.neuropharm.2012.07.047. Epub 2012 Aug 5. Neuropharmacology. 2012. PMID: 22884499 Free PMC article.
-
Is Adult Hippocampal Neurogenesis Really Relevant for the Treatment of Psychiatric Disorders?Curr Neuropharmacol. 2021;19(10):1640-1660. doi: 10.2174/1570159X18666200818194948. Curr Neuropharmacol. 2021. PMID: 32811415 Free PMC article. Review.
References
-
- Boyes B, Kim SU, Lee V, Sung SC. Immunohistochemical colocalization of S-100b and the glial fibrillary acidic protein in rat brain. Neuroscience. 1986;17:857–865. - PubMed
-
- Cameron H, McKay RDG. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–897. - PubMed
-
- Cameron H, Wooley CS, McEwen BS, Gould E. Differentiation of newly boron neurons and glia in the dentate gyrus of the adult rat. J Neurosci. 1993;56:337–344. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
