Contrasting patterns of receptive field plasticity in the hippocampus and the entorhinal cortex: an adaptive filtering approach
- PMID: 11978857
- PMCID: PMC6758357
- DOI: 10.1523/JNEUROSCI.22-09-03817.2002
Contrasting patterns of receptive field plasticity in the hippocampus and the entorhinal cortex: an adaptive filtering approach
Abstract
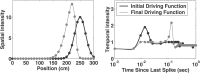
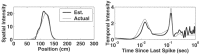
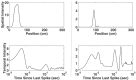
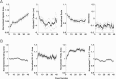
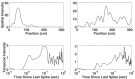
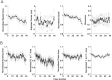
Neural receptive fields are frequently plastic: a neural response to a stimulus can change over time as a result of experience. We developed an adaptive point process filtering algorithm that allowed us to estimate the dynamics of both the spatial receptive field (spatial intensity function) and the interspike interval structure (temporal intensity function) of neural spike trains on a millisecond time scale without binning over time or space. We applied this algorithm to both simulated data and recordings of putative excitatory neurons from the CA1 region of the hippocampus and the deep layers of the entorhinal cortex (EC) of awake, behaving rats. Our simulation results demonstrate that the algorithm accurately tracks simultaneous changes in the spatial and temporal structure of the spike train. When we applied the algorithm to experimental data, we found consistent patterns of plasticity in the spatial and temporal intensity functions of both CA1 and deep EC neurons. These patterns tended to be opposite in sign, in that the spatial intensity functions of CA1 neurons showed a consistent increase over time, whereas those of deep EC neurons tended to decrease, and the temporal intensity functions of CA1 neurons showed a consistent increase only in the "theta" (75-150 msec) region, whereas those of deep EC neurons decreased in the region between 20 and 75 msec. In addition, the minority of deep EC neurons whose spatial intensity functions increased in area over time fired in a significantly more spatially specific manner than non-increasing deep EC neurons. We hypothesize that this subset of deep EC neurons may receive more direct input from CA1 and may be part of a neural circuit that transmits information about the animal's location to the neocortex.
Figures
Similar articles
-
A comparison of the firing properties of putative excitatory and inhibitory neurons from CA1 and the entorhinal cortex.J Neurophysiol. 2001 Oct;86(4):2029-40. doi: 10.1152/jn.2001.86.4.2029. J Neurophysiol. 2001. PMID: 11600659
-
Trajectory encoding in the hippocampus and entorhinal cortex.Neuron. 2000 Jul;27(1):169-78. doi: 10.1016/s0896-6273(00)00018-0. Neuron. 2000. PMID: 10939340
-
An analysis of neural receptive field plasticity by point process adaptive filtering.Proc Natl Acad Sci U S A. 2001 Oct 9;98(21):12261-6. doi: 10.1073/pnas.201409398. Proc Natl Acad Sci U S A. 2001. PMID: 11593043 Free PMC article.
-
A metric for space.Hippocampus. 2008;18(12):1142-56. doi: 10.1002/hipo.20483. Hippocampus. 2008. PMID: 19021254 Review.
-
Memory, navigation and theta rhythm in the hippocampal-entorhinal system.Nat Neurosci. 2013 Feb;16(2):130-8. doi: 10.1038/nn.3304. Epub 2013 Jan 28. Nat Neurosci. 2013. PMID: 23354386 Free PMC article. Review.
Cited by
-
Time-varying generalized linear models: characterizing and decoding neuronal dynamics in higher visual areas.Front Comput Neurosci. 2024 Jan 29;18:1273053. doi: 10.3389/fncom.2024.1273053. eCollection 2024. Front Comput Neurosci. 2024. PMID: 38348287 Free PMC article. Review.
-
Integrating Statistical and Machine Learning Approaches for Neural Classification.IEEE Access. 2022;10:119106-119118. doi: 10.1109/access.2022.3221436. Epub 2022 Nov 10. IEEE Access. 2022. PMID: 37223667 Free PMC article.
-
New experiences enhance coordinated neural activity in the hippocampus.Neuron. 2008 Jan 24;57(2):303-13. doi: 10.1016/j.neuron.2007.11.035. Neuron. 2008. PMID: 18215626 Free PMC article.
-
nSTAT: open-source neural spike train analysis toolbox for Matlab.J Neurosci Methods. 2012 Nov 15;211(2):245-64. doi: 10.1016/j.jneumeth.2012.08.009. Epub 2012 Sep 5. J Neurosci Methods. 2012. PMID: 22981419 Free PMC article.
-
CONTINUOUS-TIME FILTERS FOR STATE ESTIMATION FROM POINT PROCESS MODELS OF NEURAL DATA.Stat Sin. 2008;18(4):1293-1310. Stat Sin. 2008. PMID: 22065511 Free PMC article.
References
-
- Alonso A, Garcia-Austt E. Neuronal sources of theta rhythm in the entorhinal cortex of the rat. II. Phase relations between unit discharges and theta field potentials. Exp Brain Res. 1987;67:502–509. - PubMed
-
- Amaral DG, Witter MP. Hippocampal formation. In: Paxinos C, editor. The rat nervous system. Academic; New York: 1995. pp. 443–493.
-
- Barbieri R, Quirk MC, Frank LM, Wilson M, Brown EN. Construction and analysis of non-Poisson stimulus response models of neural spike train activity. J Neurosci Methods. 2001;105:25–37. - PubMed
-
- Barnes CA, McNaughton BL, Mizumori SJ, Leonard BW, Lin LH. Comparison of spatial and temporal characteristics of neuronal activity in sequential stages of hippocampal processing. Prog Brain Res. 1990;83:287–300. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
