WSTF-ISWI chromatin remodeling complex targets heterochromatic replication foci
- PMID: 11980720
- PMCID: PMC125993
- DOI: 10.1093/emboj/21.9.2231
WSTF-ISWI chromatin remodeling complex targets heterochromatic replication foci
Erratum in
- EMBO J 2002 Jun 17;21(12):3212
Abstract
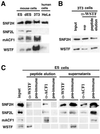
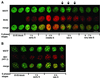
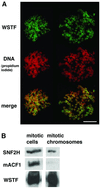
The Williams Syndrome Transcription Factor (WSTF), the product of the WBSCR9 gene, is invariably deleted in the haploinsufficiency Williams-Beuren Syndrome. Along with the nucleosome-dependent ATPase ISWI, WSTF forms a novel chromatin remodeling complex, WICH (WSTF-ISWI chromatin remodeling complex), which is conserved in vertebrates. The WICH complex was purified to homogeneity from Xenopus egg extract and was found to contain only WSTF and ISWI. In mouse cells, WSTF interacts with the SNF2H isoform of ISWI. WSTF accumulates in pericentric heterochromatin coincident with the replication of these structures, suggesting a role for WSTF in the replication of heterochromatin. Such a role is supported by the in vitro activity of both the mouse and frog WICH complexes: they are involved in the assembly of regular spaced nucleosomal arrays. In contrast to the related ISWI-interacting protein ACF1/WCRF180, WSTF binds stably to mitotic chromosomes. As dysfunction of other chromatin remodeling factors often has severe effects on development, haploinsufficiency of WSTF may explain some of the phenotypes associated with this disease.
Figures




References
-
- Aihara T., Miyoshi,Y., Koyama,K., Suzuki,M., Takahashi,E., Monden,M. and Nakamura,Y. (1998) Cloning and mapping of SMARCA5 encoding hSNF2H, a novel human homologue of Drosophila ISWI. Cytogenet. Cell Genet., 81, 191–193. - PubMed
-
- Amir R.E., Van den Veyver,I.B., Wan,M., Tran,C.Q., Francke,U. and Zoghbi,H.Y. (1999) Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nature Genet., 23, 185–188. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

