The glutamine commute: take the N line and transfer to the A
- PMID: 11980913
- PMCID: PMC2173294
- DOI: 10.1083/jcb.200201070
The glutamine commute: take the N line and transfer to the A
Abstract
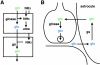
The transfer of glutamine between cells contributes to signaling as well as to metabolism. The recent identification and characterization of the system N and A family of transporters has begun to suggest mechanisms for the directional transfer of glutamine, and should provide ways to test its physiological significance in diverse processes from nitrogen to neurotransmitter release.
Figures

References
-
- Albers, A., A. Broer, C.A. Wagner, I. Setiawan, P.A. Lang, E.U. Kranz, F. Lang, and S. Broer. 2001. Na+ transport by the neural glutamine transporter ATA1. Pflugers Arch. 443:92–101. - PubMed
-
- Barnett, N.L., D.V. Pow, and S.R. Robinson. 2000. Inhibition of Müller cell glutamine synthetase rapidly impairs the retinal response to light. Glia. 30:64–73. - PubMed
-
- Bender, D.A. 1975. Amino Acid Metabolism. John Wiley & Sons Inc., London. 234 pp.
-
- Bennett, M.J., A. Marchant, H.G. Green, S.T. May, S.P. Ward, P.A. Millner, A.R. Walker, B. Schulz, and K.A. Feldmann. 1996. Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science. 273:948–950. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

