Human Speedy: a novel cell cycle regulator that enhances proliferation through activation of Cdk2
- PMID: 11980914
- PMCID: PMC2173287
- DOI: 10.1083/jcb.200109045
Human Speedy: a novel cell cycle regulator that enhances proliferation through activation of Cdk2
Abstract
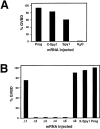
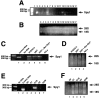
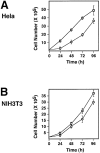
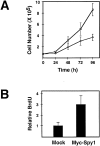
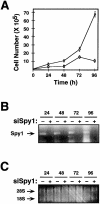
The decision for a cell to self-replicate requires passage from G1 to S phase of the cell cycle and initiation of another round of DNA replication. This commitment is a critical one that is tightly regulated by many parallel pathways. Significantly, these pathways converge to result in activation of the cyclin-dependent kinase, cdk2. It is, therefore, important to understand all the mechanisms regulating cdk2 to determine the molecular basis of cell progression. Here we report the identification and characterization of a novel cell cycle gene, designated Speedy (Spy1). Spy1 is 40% homologous to the Xenopus cell cycle gene, X-Spy1. Similar to its Xenopus counterpart, human Speedy is able to induce oocyte maturation, suggesting similar biological characteristics. Spy1 mRNA is expressed in several human tissues and immortalized cell lines and is only expressed during the G1/S phase of the cell cycle. Overexpression of Spy1 protein demonstrates that Spy1 is nuclear and results in enhanced cell proliferation. In addition, flow cytometry profiles of these cells demonstrate a reduction in G1 population. Changes in cell cycle regulation can be attributed to the ability of Spy1 to bind to and prematurely activate cdk2 independent of cyclin binding. We demonstrate that Spy1-enhanced cell proliferation is dependent on cdk2 activation. Furthermore, abrogation of Spy1 expression, through the use of siRNA, demonstrates that Spy1 is an essential component of cell proliferation pathways. Hence, human Speedy is a novel cell cycle protein capable of promoting cell proliferation through the premature activation of cdk2 at the G1/S phase transition.
Figures








Similar articles
-
Speedy: a novel cell cycle regulator of the G2/M transition.EMBO J. 1999 Apr 1;18(7):1869-77. doi: 10.1093/emboj/18.7.1869. EMBO J. 1999. PMID: 10202150 Free PMC article.
-
Spy1 interacts with p27Kip1 to allow G1/S progression.Mol Biol Cell. 2003 Sep;14(9):3664-74. doi: 10.1091/mbc.e02-12-0820. Epub 2003 Jul 11. Mol Biol Cell. 2003. PMID: 12972555 Free PMC article.
-
Human Spy1 promotes survival of mammalian cells following DNA damage.Cancer Res. 2003 Jul 1;63(13):3701-7. Cancer Res. 2003. PMID: 12839962
-
[Cell cycle control in mammalian cells].Seikagaku. 1994 Feb;66(2):126-36. Seikagaku. 1994. PMID: 8163865 Review. Japanese. No abstract available.
-
RINGO/Speedy proteins, a family of non-canonical activators of CDK1 and CDK2.Semin Cell Dev Biol. 2020 Nov;107:21-27. doi: 10.1016/j.semcdb.2020.03.010. Epub 2020 Apr 18. Semin Cell Dev Biol. 2020. PMID: 32317145 Review.
Cited by
-
The cyclin-like protein Spy1/RINGO promotes mammary transformation and is elevated in human breast cancer.BMC Cancer. 2012 Jan 26;12:45. doi: 10.1186/1471-2407-12-45. BMC Cancer. 2012. PMID: 22280365 Free PMC article.
-
Revealed: The spy who regulates neuroblastoma stem cells.Oncotarget. 2014 Nov 30;5(22):11014-6. doi: 10.18632/oncotarget.2839. Oncotarget. 2014. PMID: 25483101 Free PMC article.
-
Upregulation of the cell-cycle regulator RGC-32 in Epstein-Barr virus-immortalized cells.PLoS One. 2011;6(12):e28638. doi: 10.1371/journal.pone.0028638. Epub 2011 Dec 6. PLoS One. 2011. PMID: 22163048 Free PMC article.
-
Biochemical characterization of Cdk2-Speedy/Ringo A2.BMC Biochem. 2005 Sep 28;6:19. doi: 10.1186/1471-2091-6-19. BMC Biochem. 2005. PMID: 16191191 Free PMC article.
-
Female Germ Cell Development in Chickens and Humans: The Chicken Oocyte Enriched Genes Convergent and Divergent with the Human Oocyte.Int J Mol Sci. 2022 Sep 27;23(19):11412. doi: 10.3390/ijms231911412. Int J Mol Sci. 2022. PMID: 36232712 Free PMC article. Review.
References
-
- Bartek, J., J. Bartkova, and J. Lukas. 1996. The retinoblastoma protein pathway and the restriction point. Curr. Opin. Cell Biol. 8:805–814. - PubMed
-
- Ekholm, S.V., and S.I. Reed. 2000. Regulation of G(1) cyclin dependent kinases in the mammalian cell cycle. Curr. Opin. Cell Biol. 12:676–684. - PubMed
-
- Fang, F., and J.W. Newport. 1991. Evidence that the G1-S and G2-M transitions are controlled by different cdc2 proteins in higher eukaryotes. Cell. 66:731–742. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

