DAP kinase and DRP-1 mediate membrane blebbing and the formation of autophagic vesicles during programmed cell death
- PMID: 11980920
- PMCID: PMC2173279
- DOI: 10.1083/jcb.200109094
DAP kinase and DRP-1 mediate membrane blebbing and the formation of autophagic vesicles during programmed cell death
Abstract
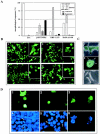
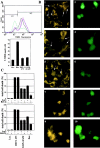
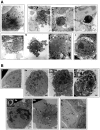
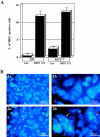
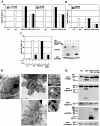
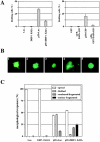
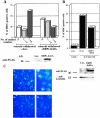
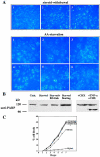
Death-associated protein kinase (DAPk) and DAPk-related protein kinase (DRP)-1 proteins are Ca+2/calmodulin-regulated Ser/Thr death kinases whose precise roles in programmed cell death are still mostly unknown. In this study, we dissected the subcellular events in which these kinases are involved during cell death. Expression of each of these DAPk subfamily members in their activated forms triggered two major cytoplasmic events: membrane blebbing, characteristic of several types of cell death, and extensive autophagy, which is typical of autophagic (type II) programmed cell death. These two different cellular outcomes were totally independent of caspase activity. It was also found that dominant negative mutants of DAPk or DRP-1 reduced membrane blebbing during the p55/tumor necrosis factor receptor 1-induced type I apoptosis but did not prevent nuclear fragmentation. In addition, expression of the dominant negative mutant of DRP-1 or of DAPk antisense mRNA reduced autophagy induced by antiestrogens, amino acid starvation, or administration of interferon-gamma. Thus, both endogenous DAPk and DRP-1 possess rate-limiting functions in these two distinct cytoplasmic events. Finally, immunogold staining showed that DRP-1 is localized inside the autophagic vesicles, suggesting a direct involvement of this kinase in the process of autophagy.
Figures

References
-
- Anglade, P., S. Vyas, F. Javoy-Agid, M.T. Herrero, P.P. Michel, J. Marquez, A. Mouatt-Prigent, M. Ruberg, E.C. Hirsch, and Y. Agid. 1997. Apoptosis and autophagy in nigral neurons of patients with Parkinson's disease. Histol. Histopathol. 12:25–31. - PubMed
-
- Biederbick, A., H.F. Kern, and H.P. Elsasser. 1995. Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur. J. Cell Biol. 66:3–14. - PubMed
-
- Bursch, W., A. Ellinger, H. Kienzl, L. Torok, S. Pandey, M. Sikorska, R. Walker, and R.S. Hermann. 1996. Active cell death induced by the anti-estrogens tamoxifen and ICI 164 384 in human mammary carcinoma cells (MCF-7) in culture: the role of autophagy. Carcinogenesis. 17:1595–1607. - PubMed
-
- Bursch, W., K. Hochegger, L. Torok, B. Marian, A. Ellinger, and R.S. Hermann. 2000. Autophagic and apoptotic types of programmed cell death exhibit different fates of cytoskeletal filaments. J. Cell Sci. 113:1189–1198. - PubMed
-
- Cataldo, A.M., D.J. Hamilton, and R.A. Nixon. 1994. Lysosomal abnormalities in degenerating neurons link neuronal compromise to senile plaque development in Alzheimer disease. Brain Res. 640:68–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

