Distinguishing cytomegalovirus (CMV) infection and disease with CMV nucleic acid assays
- PMID: 11980925
- PMCID: PMC130923
- DOI: 10.1128/JCM.40.5.1581-1586.2002
Distinguishing cytomegalovirus (CMV) infection and disease with CMV nucleic acid assays
Abstract
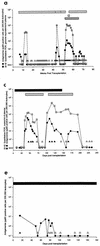
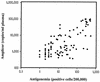
Human cytomegalovirus (CMV) continues to be a significant cause of morbidity and mortality among transplant recipients. Molecular assays have been developed for the detection and quantification of CMV nucleic acid. In evaluating the clinical utility of these assays, correlations with clinical outcome are essential. The Amplicor CMV Monitor and NucliSens CMV pp67 tests were compared to the CMV antigenemia assay for 45 transplant recipients and 1 patient with Wegener's granulomatosis. Twenty-three patients remained antigenemia negative throughout the monitoring period, none of whom developed CMV disease. In this patient group, both the Amplicor and NucliSens assays showed very high specificity; only 1 of the 324 specimens assayed by NucliSens and none of the 303 specimens assayed by Amplicor were positive. Twenty-three patients were antigenemia positive during the monitoring period, 12 of whom developed 13 episodes of symptomatic CMV disease. In this patient group, the NucliSens assay was positive at or before the development of symptoms in 12 of the 13 episodes of CMV disease. All eight patients with symptomatic CMV disease who were tested by the Amplicor assay were positive at or before the development of disease. For the 11 asymptomatic patients, the NucliSens assay was positive less frequently than the antigenemia or Amplicor assays. The NucliSens assay was more likely to be positive at higher antigenemia or viral load levels. Both the NucliSens and Amplicor assays appear to have clinical utility in monitoring patients for CMV disease.
Figures


References
-
- Blok, M. J., V. J. Goossens, J. V. Vanherle, B. Top, N. Tacken, J. M. Middeldorp, M. H. L. Christiaans, J. P. van Hooff, and C. A. Bruggeman. 1998. Diagnostic value of monitoring human cytomegalovirus late pp67 mRNA expression in renal-allograft recipients by nucleic acid sequence-based amplification. J. Clin. Microbiol. 36:1341-1346. - PMC - PubMed
-
- Blok, M. J., I. Lautenschlager, V. J. Goossens, J. M. Middeldorp, C. Vink, K. Hockerstedt, and C. A. Bruggeman. 2000. Diagnostic implications of human cytomegalovirus immediate early-1 and pp67 mRNA detection in whole-blood samples from liver transplant patients using nucleic acid sequenced-based amplification. J. Clin. Microbiol. 38:4485-4491. - PMC - PubMed
-
- Caliendo, A. M., K. St. George, S.-Y. Kao, J. Allega, B.-H. Tan, R. LaFontaine, L. Bui, and C. R. Rinaldo. 2000. Comparison of quantitative cytomegalovirus (CMV) PCR in plasma and CMV antigenemia assay: clinical utility of the prototype AMPLICOR CMV MONITOR test in transplant recipients. J. Clin. Microbiol. 38:2122-2127. - PMC - PubMed
-
- Conti, D. J., B. M. Freed, T. P. Singh, M. Gallichio, S. A. Gruber, and N. Lempert. 1995. Preemptive ganciclovir therapy in cytomegalovirus-seropositive renal transplant recipients. Arch. Surg. 130:1217-1222. - PubMed

