Effects of aldosterone on biosynthesis, traffic, and functional expression of epithelial sodium channels in A6 cells
- PMID: 11981022
- PMCID: PMC2233818
- DOI: 10.1085/jgp.20028559
Effects of aldosterone on biosynthesis, traffic, and functional expression of epithelial sodium channels in A6 cells
Abstract
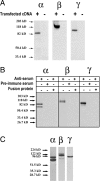
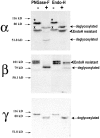
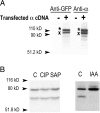
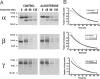
The collecting duct regulates Na(+) transport by adjusting the abundance/activity of epithelial Na(+) channels (ENaC). In this study we have investigated the synthesis, degradation, endocytosis, and activity of ENaC and the effects of aldosterone on these processes using endogenous channels expressed in the A6 cell line. Biochemical studies were performed with a newly raised set of specific antibodies against each of the three subunits of the amphibian ENaC. Our results indicate simultaneous transcription and translation of alpha, beta, and gamma subunits and enhancement of both processes by aldosterone: two- and fourfold increase, respectively. The biosynthesis of new channels can be followed by acquisition of endoglycosidase H-resistant oligosacharides in alpha and beta subunits and, in the case of alpha, by the appearance of a form resistant to reducing agents. The half-life of the total pool of subunits (t(1/2) 40-70 min) is longer than the fraction of channels in the apical membrane (t(1/2) 12-17 min). Aldosterone induces a fourfold increase in the abundance of the three subunits in the apical membrane without significant changes in the open probability, kinetics of single channels, or in the rate of degradation of ENaC subunits. Accordingly, the aldosterone response could be accounted by an increase in the abundance of apical channels due, at least in part, to de novo synthesis of subunits.
Figures
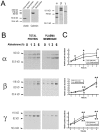

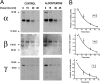




Comment in
-
Hormonal regulation of the epithelial sodium channel ENaC: N or P(o)?J Gen Physiol. 2002 Jul;120(1):67-70. doi: 10.1085/jgp.20028638. J Gen Physiol. 2002. PMID: 12084776 Free PMC article. Review. No abstract available.
References
-
- Alvarez de la Rosa, D., P. Zhang, A. Naray-Fejes-Toth, G. Fejes-Toth, and C.M. Canessa. 1999. The serum and glucocorticoid kinase sgk increases the abundance of epithelial sodium channels in the plasma membrane of Xenopus oocytes. J. Biol. Chem. 274:37834–37839. - PubMed
-
- Alvarez de la Rosa, D., C.M. Canessa, G.K. Fyfe, and P. Zhang. 2000. Structure and regulation of amiloride-sensitive sodium channels. Annu. Rev. Physiol. 62:573–594. - PubMed
-
- Asher, C., H. Wald, B.C. Rossier, and H. Garty. 1996. Aldosterone-induced increase in the abundance of Na+ channel subunits. Am. J. Physiol. 271:C605–C611. - PubMed
-
- Bindels, R.J., J.A. Schafer, and M.C. Reif. 1988. Stimulation of sodium transport by aldosterone and arginine vasotocin in A6 cells. Biochim. Biophys. Acta. 972:320–330. - PubMed

