Openings of the rat recombinant alpha 1 homomeric glycine receptor as a function of the number of agonist molecules bound
- PMID: 11981023
- PMCID: PMC2233816
- DOI: 10.1085/jgp.20028530
Openings of the rat recombinant alpha 1 homomeric glycine receptor as a function of the number of agonist molecules bound
Abstract
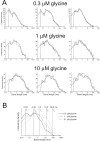
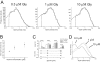
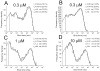
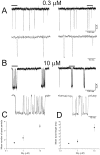
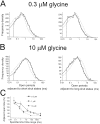
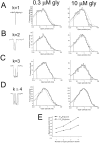
The functional properties of rat homomeric alpha 1 glycine receptors were investigated using whole-cell and outside-out recording from human embryonic kidney cells transfected with rat alpha1 subunit cDNA. Whole-cell dose-response curves gave EC(50) estimates between 30 and 120 microM and a Hill slope of approximately 3.3. Single channel recordings were obtained by steady-state application of glycine (0.3, 1, or 10 microM) to outside-out patches. Single channel conductances were mostly 60-90 pS, but smaller conductances of approximately 40 pS were also seen (10% of the events) with a relative frequency that did not depend on agonist concentration. The time constants of the apparent open time distributions did not vary with agonist concentration, but short events were more frequent at low glycine concentrations. There was also evidence of a previously missed short-lived open state that was more common at lower glycine concentrations. The time constants for the different components of the burst length distributions were found to have similar values at different concentrations. Nevertheless, the mean burst length increased with increasing glycine. This was because the relative area of each burst-length component was concentration dependent and short bursts were favored at lower glycine concentrations. Durations of adjacent open and shut times were found to be strongly (negatively) correlated. Additionally, long bursts were made up of longer than average openings separated by short gaps, whereas short bursts usually consisted of single isolated short openings. The most plausible explanation for these findings is that long bursts are generated when a higher proportion of the five potential agonist binding sites on the receptor is occupied by glycine. On the basis of the concentration dependence and the intraburst structure we provide a preliminary kinetic scheme for the activation of the homomeric glycine receptor, in which any number of glycine molecules from one to five can open the channel, although not with equal efficiency.
Figures










References
-
- Akagi, H., K. Hirai, and F. Hishinuma. 1991. Functional properties of strychnine-sensitive glycine receptors expressed in Xenopus oocytes injected with a single mRNA. Neurosci. Res. 11:28–40. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

