Recall and propagation of allospecific memory T cells independent of secondary lymphoid organs
- PMID: 11983909
- PMCID: PMC122922
- DOI: 10.1073/pnas.092596999
Recall and propagation of allospecific memory T cells independent of secondary lymphoid organs
Abstract
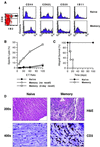
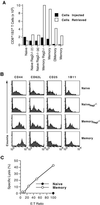
The allospecifc T cell population responding to a transplanted organ consists of both naive and memory lymphocytes. Although it is established that naive T cells are activated by antigen within the organized structures of secondary lymphoid organs (the spleen, lymph nodes, and mucosal lymphoid tissues), it is not clear whether memory T cell activation and propagation depend on homing to these organs. To answer this question, we investigated whether allospecific naive or memory T cells can mediate acute cardiac allograft rejection in mutant mice that lack all of their secondary lymphoid tissues. The results of our experiments demonstrated that antigen-experienced memory T cells have two advantages over naive T cells: (i) memory T cells mount a vigorous immune response that leads to allograft rejection independent of secondary lymphoid organs; and (ii) memory T cells generate more memory T cells without homing to secondary lymphoid organs. These unique properties of memory T cells were further confirmed by showing that memory-like T cells that arise from the homeostatic proliferation of naive T cells in the absence of antigenic stimulation are suboptimal at rejecting allografts and do not generate memory T cells in mice devoid of secondary lymphoid tissues.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

