Tissue-specific expression of mast cell granule serine proteinases and their role in inflammation in the lung and gut
- PMID: 11985658
- PMCID: PMC1782685
- DOI: 10.1046/j.1365-2567.2002.01375.x
Tissue-specific expression of mast cell granule serine proteinases and their role in inflammation in the lung and gut
Abstract
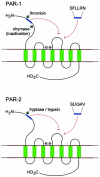
Serine proteinases with trypsin-like (tryptase) and chymotrypsin-like (chymase) properties are major constituents of mast cell granules. Several tetrameric tryptases with differing specificities have been characterized in humans, but only a single chymase. In other species there are larger families of chymases with distinct and narrow proteolytic specificities. Expression of chymases and tryptases varies between tissues. Human pulmonary and gastrointestinal mast cells express chymase at lower levels than tryptase, whereas rodent and ruminant gastrointestinal mast cells express uniquely mucosa-specific chymases. Local and systemic release of chymases and tryptases can be quantified by immunoassay, providing highly specific markers of mast cell activation. The expression and constitutive extracellular secretion of the mucosa-specific chymase, mouse mast cell proteinase-1 (mMCP-1), is regulated by transforming growth factor-beta1 (TGF-beta1) in vitro, but it is not clear how the differential expression of chymases and tryptases is regulated in other species. Few native inhibitors have been identified for tryptases but the tetramers dissociate into inactive subunits in the absence of heparin. Chymases are variably inhibited by plasma proteinase inhibitors and by secretory leucocyte protease inhibitor (SLPI) that is expressed in the airways. Tryptases and chymases promote vascular permeability via indirect and possibly direct mechanisms. They contribute to tissue remodelling through selective proteolysis of matrix proteins and through activation of proteinase-activated receptors and of matrix metalloproteinases. Chymase may modulate vascular tissues through its ability to process angiotensin-I to angiotensin-II. Mucosa-specific chymases promote epithelial permeability and are involved in the immune expulsion of intestinal nematodes. Importantly, granule proteinases released extracellularly contribute to the recruitment of inflammatory cells and may thus be involved in innate responses to infection.
Figures

References
-
- Miller HR. Mucosal mast cells and the allergic response against nematode parasites. Vet Immunol Immunopathol. 1996;54:331–6. - PubMed
-
- Enerback L. Mucosal mast cells in the rat and in man. Int Arch Allergy Appl Immunol. 1987;82:249–55. - PubMed
-
- Befus D. Intestinal mast cell polymorphism: new research directions and clinical implications. J Pediatr Gastroenterol Nutr. 1986;5:517–21. - PubMed
-
- Irani AM, Schwartz LB. Human mast cell heterogeneity. Allergy Proc. 1994;15:303–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

